
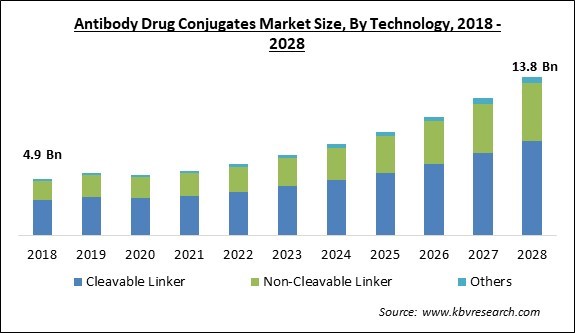
The Global Antibody Drug Conjugates Market size is expected to reach $13.8 billion by 2028, rising at a market growth of 14.2% CAGR during the forecast period.
Antibody Drug Conjugates (ADCs) are biopharmaceutical drugs that are particularly effective in the treatment of cancer. ADCs are designed to kill cancer cells while leaving healthy cells alone. Antibody-drug conjugates (ADCs) are a new class of anti-cancer medications that have been created in recent years. ADCs have shown to be a good substitute for conventional cancer treatments like chemotherapy or combination therapy because of their low off-target effects and significant cytotoxicity at tumor locations. Several advances in antibody-drug engineering have resulted in very effective tumor-targeting medicines with a broad therapeutic window. Two ADCs are already on the market such as Brentuximab vedotin and Trastuzumab emtansine, and many others are in clinical development. Enzymes in conjunction with prodrugs have proven to be a viable option. Horseradish peroxidase (HRP), a glyco-enzyme, has been shown to convert the hormone indole-3-acetic acid (IAA) into a highly effective cytotoxic poison. The utilization of this mixture of IAA and HRP in techniques like gene-directed enzyme prodrug treatment (GDEPT) and antibody-directed enzyme prodrug therapy (ADEPT) has been studied (ADEPT).
For instance, the market is likely to rise due to increased investment in this industry by key players like Pfizer Inc., Piramal Pharma Solution, F. Hoffman La-Roche, and Seagen, for the development of novel ADCs for cancer treatment. Additionally, for example, to meet the increased demand for commercial ADCs in the UK, CDMO Piramal Pharma Solutions (PPS) committed USD 74.4 million to the formation of two modern antibody-drug conjugates (ADC) production facilities at its present sites in Grangemouth, Scotland in February 2022.
Clinical trials are being conducted by the majority of corporations in order to move new products to market and to achieve label expansion for products that have previously been approved. Seagen, for example, began phase 1 clinical trials of two new antibody-drug conjugates, SGN-B7H4V and SGN-PDL1V, in patients with advanced solid cancers in January 2022. Additionally, the business, in conjunction with Astellas, finished patient recruitment for the cohort K EV-103 trial, which is used to treat First-Line Metastatic Urothelial Cancer (mUC). Market expansion is likely to be fueled by the satisfactory completion of the clinical trial research and future product approval.
With the overall COVID-19 pandemic, the expansion of many industries saw a decline in demand, however, several other areas remained untouched and showed promising indicators of future growth. COVID-19 impacted several businesses in different ways. As activities within hospitals and healthcare facilities were severely limited due to social distancing and lockdown measures enacted by governments around the world, the breakout of the COVID-19 pandemic had a massive impact on the antibody-drug conjugates business.
The rise in incidence of cancer within the population is a serious medical condition which is occurring due to various unknown reasons, and this have raised a substantial need of an advanced medical equipment to treat and cure various types of cancer diseases. Equipment in support is used extensively in companion diagnostics, customized pharmaceuticals, and other disease diagnostics, like illness risk assessment and drug research and development. The growing use of biomarkers in disease detection is one of the major factors anticipated to enhance antibody-drug conjugates market growth in the future years. The Lung and breast cancer are more existing cancers recorded since past few years.
Emerging country governments are increasing the expenditures in the healthcare sector. Governments around the world plan to enhance their health systems with this investment in order to deliver better and more modern solutions and minimize chronic disease cases. An ageing population, an increasing middle class, a growing proportion of lifestyle diseases, enhanced focus on public-private partnerships, fasten adoption of digital technologies, comprising of telemedicine, as well as enhanced investor interest and FDI inflows across the last two decades, are all boosting the growth of the healthcare infrastructure.
The exorbitant cost of cancer therapy is the most critical challenge facing the antibody-drug conjugates market. Prices for cancer medicines are rising in lockstep with their improving success rates. Cancer drugs can be expensive on a monthly basis, which the majority of people cannot manage. Even the wealthy, leave the poor or middle classes, struggle to bear the price of such costly therapies. The cost of medication may be multiple times what many people earn in a month. The rising price of cancer diagnosis and treatment in poor nations is another major impediment to the market's growth. Patients are increasingly selecting various low-cost detection techniques, which is limiting the number of patients who are examined for the concerned purpose.
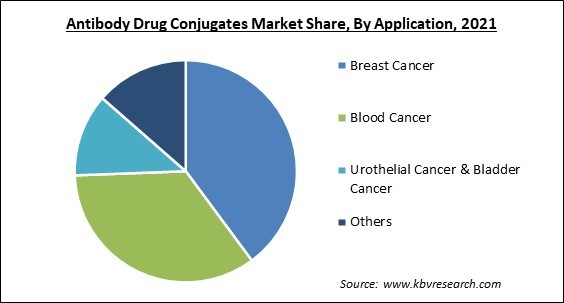
Based on Application, the market is segmented into Breast Cancer, Blood Cancer, Urothelial Cancer & Bladder Cancer, and Others. The blood cancer segment garnered a significant revenue share in the antibody-drug conjugates market in 2021. Blood cancer is the fifth most frequent malignancy worldwide and the second main cause of death from cancer. For instance, as per the National Foundation for Cancer Research, 186,400 new instances of leukemia, lymphoma, and myeloma will be diagnosed in the United States in 2021. Additionally, several product approvals would benefit the market.
Based on Technology, the market is segmented into Cleavable Linker, Non-Cleavable Linker, and Others. The cleavable linkers segment acquired the highest revenue share in the antibody-drug conjugates market in 2021. Cleavable linkers are the most widely employed technology for an effective outcome of ADCs in cancer treatment because of the ability to stay stable in the bloodstream for longer periods of time and release a cytotoxin from ADCs.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 5.7 Billion |
| Market size forecast in 2028 | USD 13.8 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 14.2% from 2022 to 2028 |
| Number of Pages | 188 |
| Number of Tables | 273 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Technology, Application, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. North America emerged as the leading region in the antibody-drug conjugates market with the largest revenue share in 2021. The region's supremacy is due to well-established research facilities for the creation of novel ADCs, increasing per capita healthcare expenditure, and a rise in the prevalence of cancer. For instance, according to the American Cancer Society, cancer will affect 1.9 million people in the United States in 2022, resulting in 609,360 deaths. Market growth is likely to be fueled by the approval of new ADCs in the region. For example, the FDA authorized Tidvak in September 2021 for the treatment of adult patients with recurrent cervical or metastatic cancer.
Free Valuable Insights: Global Antibody Drug Conjugates Market size to reach USD 13.8 Billion by 2028
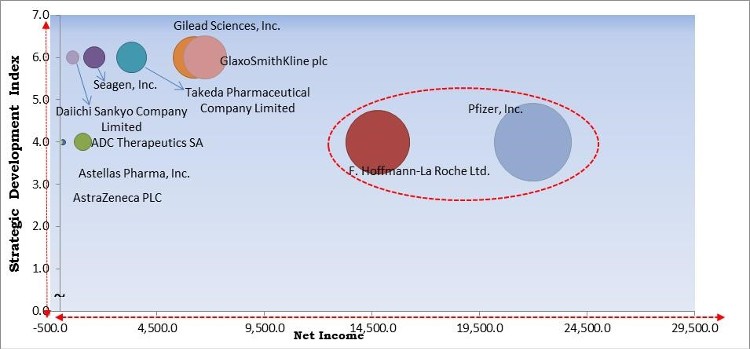
The major strategies followed by the market participants are Partnerships. Based on the Analysis presented in the Cardinal matrix; F. Hoffmann-La Roche Ltd. and Pfizer, Inc. are the forerunners in the Antibody Drug Conjugates Market. Companies such as GlaxoSmithKline plc, Gilead Sciences, Inc., Takeda Pharmaceutical Company Limited are some of the key innovators in the Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Takeda Pharmaceutical Company Limited, F. Hoffmann-La Roche Ltd., AstraZeneca PLC, GlaxoSmithKline PLC, Pfizer, Inc., Astellas Pharma, Inc., Gilead Sciences, Inc., Seagen, Inc., ADC Therapeutics SA, and Daiichi Sankyo Company, Limited.
By Application
By Technology
By Geography
The antibody drug conjugates market size is projected to reach USD 13.8 billion by 2028.
The increasing cases of cancer are increasing are driving the market in coming years, however, greater cost in the treatment of cancer growth of the market.
Takeda Pharmaceutical Company Limited, F. Hoffmann-La Roche Ltd., AstraZeneca PLC, GlaxoSmithKline PLC, Pfizer, Inc., Astellas Pharma, Inc., Gilead Sciences, Inc., Seagen, Inc., ADC Therapeutics SA, and Daiichi Sankyo Company, Limited.
Yes, COVID-19 impacted several businesses in different ways. As activities within hospitals and healthcare facilities were severely limited due to social distancing and lockdown measures enacted by governments around the world, the breakout of the pandemic had a massive impact on the antibody-drug conjugates business.
The Breast Cancer segment acquired maximum revenue share in the Global Antibody Drug Conjugates Market by Application in 2021, thereby, achieving a market value of $5.3 billion by 2028.
The North America market dominated the Global Antibody Drug Conjugates Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $5.3 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
