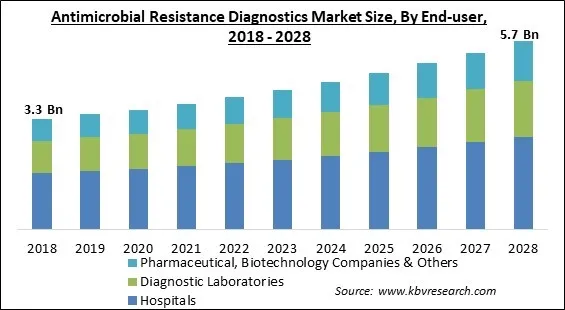
The Global Antimicrobial Resistance Diagnostics Market size is expected to reach $5.7 billion by 2028, rising at a market growth of 6.1% CAGR during the forecast period.
Antimicrobial medicines are given to combat the negative effects of microbes for a healthy life, but the overuse of these drugs leads to the development of resistance towards these medicines. This resistance is commonly termed antimicrobial resistance. The formation of resistant microbes is a natural occurrence, but the excessive or improper use of antimicrobials has significantly influenced the evolution of resistance.
Antimicrobial resistance has become the biggest danger to public health and is considered a global burden. Effective treatment plans can be guided by rapid diagnostic tests that pinpoint microorganisms with a high risk of developing drug resistance, assess antimicrobial susceptibility, and separate viral from bacterial infections. Rapid diagnostic tests also make epidemiological surveillance easier by allowing for the monitoring of developing resistant infectious pathogens and their transmission.
In order to survive, bacteria can create mechanisms known as resistance against antibiotics and antifungals. The germ's resistance mechanisms are determined by the specific proteins that DNA instructs the germ to produce. Many different forms of resistance genes can be found in bacteria and fungi. The perfect confluence of resistance mechanisms in bacteria that are already difficult to treat can render all antibiotics and antifungals useless, leading to illnesses that are untreatable. Unsettlingly, bacteria that are resistant to antibiotics and antifungals might exchange such resistance mechanisms with some other bacteria.
The COVID-19 pandemic exacerbated the ongoing antimicrobial resistance global crisis. This was because of the increased utilization of antibiotics to diagnose COVID-19 patients, disruptions to infection control and prevention procedures in overburdened health systems, and the diversion of financial and human resources away from surveilling and reacting to AMR threats. Because of COVID-19, the worldwide lockdown has made virtual visits and quick diagnostic testing necessary to help patients avoid going to the hospital. Even after the pandemic, it is projected that home-based healthcare will remain a top priority, especially for individuals with pre-existing diseases, which has facilitated the demand for diagnostic assays. Therefore, the pandemic had an overall positive impact on the antimicrobial resistance diagnostics market.
Some brand-new, speedy image-based technologies can deliver information in a clinical situation more swiftly. The prevailing culture-based techniques that necessitate isolated colonies to increase before susceptibility data could be obtained can take up to 48 hours or more. New methodologies that reveal drug resistance without the use of conventional culture procedures can reduce that time frame by at minimum one day and possibly more.
Nosocomially acquired illnesses that are not existing or hatching at the moment of admission to a hospital are known as hospital-acquired infections (HAIs). These infections may include bloodstream infections that are joined to central lines, or urinary tract infections caused by use of catheters, many surgical site infections, ventilators influenced pneumonia, or pneumonia acquired in hospitals, and infections with CD. Many different intrusive techniques and equipment are used in modern healthcare to cure patients and aid in their recovery.
The majority of commercially available quick tests for detecting antimicrobial resistance include genotypic tests, which rely on finding gene products or resistance genes. These methods have a number of drawbacks. The first is that while genotypic approaches can be useful for anticipating antibiotic resistance, they do not provide information on antimicrobial susceptibility. Also, these methods only give what is being looked for that is, typically a relatively small panel of resistance determinants is evaluated.
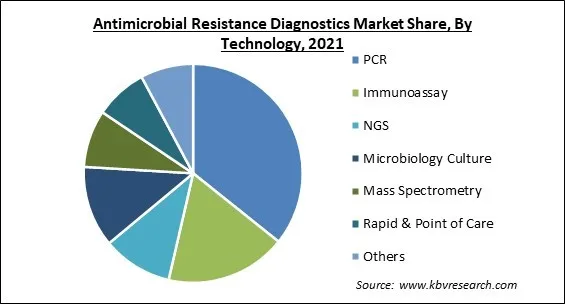
Based on technology, the antimicrobial resistance diagnostics market is categorized into microbiology culture, immunoassay, PCR, NGS, mass spectroscopy, rapid & point of care, and others. The PCR segment dominated the revenue share in the antimicrobial resistance diagnostics market in 2021. The main elements leading to the high sector share include developments in PCR technology and PCR technology's wide penetration because of its excellent accuracy. The segment is also growing as a result of PCR diagnostics for hospital-acquired infections (HAIs) like C. difficile and MRSA being used more frequently.
Based on end user, the antimicrobial resistance diagnostics market is segmented into hospitals, diagnostic laboratories, pharmaceutical & biotechnology companies, and others. The diagnostic laboratories garnered a remarkable growth rate in the antimicrobial resistance diagnostics market in 2021. It is anticipated that the growing prevalence of infectious disorders and laboratory technical developments will fuel segment expansion. Additionally, high penetration of diagnostic-focused laboratories and reasonably priced services are expected to support market growth.
On the basis of pathogen, the antimicrobial resistance diagnostics market is divided into drug resistant streptococcus pneumonia (DRSP), drug resistant campylobacter (DRC), clostridium difficile (CD), methicillin resistant staphylococcus aureus (MRSA), drug resistant Neisseria gonorrhoeae (DRNG), drug resistant salmonella (DRNTS), and others. The methicillin resistant staphylococcus aureus (MRSA) segment garnered the highest revenue share in the antimicrobial resistance diagnostics market in 2021. The primary driver of segment expansion is the rising prevalence of MRSA in hospital settings. Additionally, a rise in the number of authorizations for diagnostic tests to find MRSA is another driver causing the market to grow.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 3.8 Billion |
| Market size forecast in 2028 | USD 5.7 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 6.1% from 2022 to 2028 |
| Number of Pages | 289 |
| Number of Tables | 433 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Technology, Pathogen, End-user, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
On the basis of region, the antimicrobial resistance diagnostics market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America segment procured the maximum revenue share in the antimicrobial resistance diagnostics market in 2021. The high danger of developing antibiotic resistance, favorable government policies to address AMR, and the existence of cutting-edge healthcare infrastructure are all factors contributing to the rise of the North American region.
Free Valuable Insights: Global Antimicrobial Resistance Diagnostics Market size to reach USD 5.7 Billion by 2028
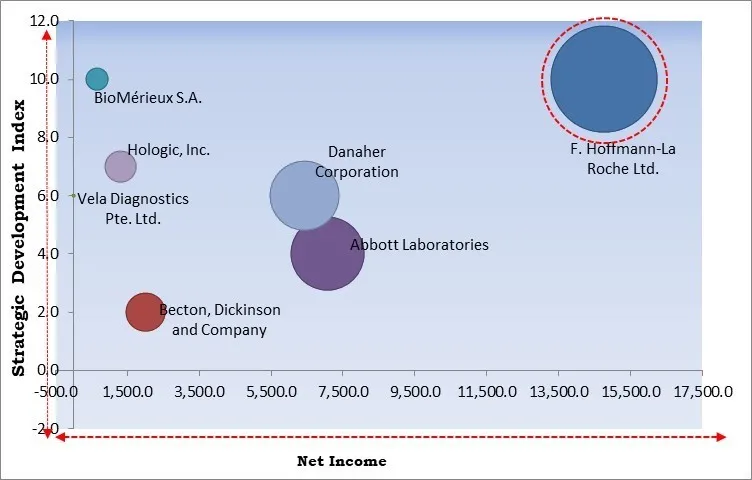
The major strategies followed by the market participants are Partnerships. Based on the Analysis presented in the Cardinal matrix; F. Hoffmann-La Roche Ltd. is the forerunner in the Antimicrobial Resistance Diagnostics Market. Companies such as Abbott Laboratories, Danaher Corporation, BioMérieux S.A. are some of the key innovators in Antimicrobial Resistance Diagnostics Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Abbott Laboratories, BioMérieux S.A., F. Hoffmann-La Roche Ltd., Becton, Dickinson and Company, Danaher Corporation, Accelerate Diagnostics, Inc., Hologic, Inc., Visby Medical, Inc., Vela Diagnostics Pte. Ltd. (Luye Medical Group Co., Ltd) and Molsid S.A.S.
By Technology
By End User
By Pathogen
By Geography
The global Antimicrobial Resistance Diagnostics Market size is expected to reach $5.7 billion by 2028.
Availability of Better Diagnostics Tests and Kits are driving the market in coming years, however, Limited Panel of Resistance Determinants Offered by Current Tests restraints the growth of the market.
Abbott Laboratories, BioMérieux S.A., F. Hoffmann-La Roche Ltd., Becton, Dickinson and Company, Danaher Corporation, Accelerate Diagnostics, Inc., Hologic, Inc., Visby Medical, Inc., Vela Diagnostics Pte. Ltd. (Luye Medical Group Co., Ltd) and Molsid S.A.S.
The expected CAGR of the Antimicrobial Resistance Diagnostics Market is 6.1% from 2022 to 2028.
The Hospitals market is leading the segment in the Global Antimicrobial Resistance Diagnostics Market by End-user in 2021, achieving a market value of $2.8 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

