The Asia Pacific Pharmaceutical Sterility Testing Market would witness market growth of 11.8% CAGR during the forecast period (2023-2030).
The China market dominated the Asia Pacific Pharmaceutical Sterility Testing Market by Country in 2022 and would continue to be a dominant market till 2030; thereby, achieving a market value of $182.2 Million by 2030. The Japan market is registering a CAGR of 11.1% during (2023 - 2030). Additionally, The India market would showcase a CAGR of 12.5% during (2023 - 2030).
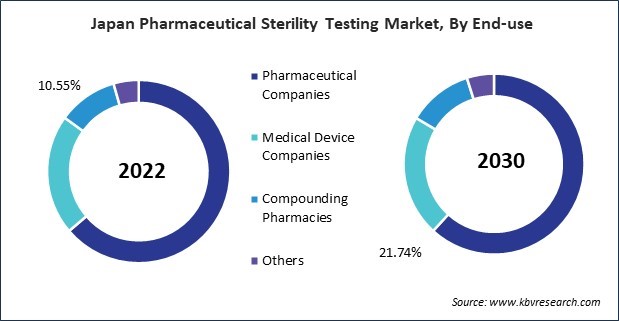
Pharmaceutical companies conduct sterility testing on incoming raw materials, such as active pharmaceutical ingredients (APIs) and excipients, to identify potential sources of contamination early in the manufacturing process. In addition, the sterility of manufacturing environments is closely monitored through environmental sampling. This proactive approach helps identify and rectify potential sources of microbial contamination in cleanrooms and production areas. Likewise, sterility testing is integral to process validation, ensuring that manufacturing processes consistently produce sterile products. This is crucial for maintaining product quality and compliance with regulatory requirements.
Furthermore, the pharmaceutical sterility testing market is witnessing dynamic trends driven by evolving regulatory landscapes, technological advancements, and industry efforts to streamline testing processes. Understanding these trends is essential for pharmaceutical companies, testing laboratories, and stakeholders in the sterility testing market. Environmental monitoring within manufacturing facilities is gaining prominence as a preventive measure. Continuous monitoring of cleanrooms and production areas helps identify potential sources of contamination, allowing for prompt corrective actions.
The medical device sector in China is subject to regulatory requirements to ensure the safety and efficacy of products. Sterility testing is a critical component of quality control and regulatory compliance. As the medical device industry expands, the demand for pharmaceutical sterility testing services will likely increase to meet stringent regulatory standards. China has become a major player in the global medical device manufacturing landscape. Hence, the rising medical device sector in the Asia Pacific will fuel the region's demand for pharmaceutical sterility testing.
Free Valuable Insights: The Global Pharmaceutical Sterility Testing Market is Predict to reach USD 3.1 Billion by 2030, at a CAGR of 10.7%
Based on Type, the market is segmented into Outsourcing, and In-House. Based on Product Type, the market is segmented into Kits & Reagents, Instruments, and Services. Based on Sample, the market is segmented into Pharmaceuticals, Medical Devices, and Biopharmaceuticals. Based on End-use, the market is segmented into Pharmaceutical Companies, Medical Device Companies, Compounding Pharmacies, and Others. Based on Test Type, the market is segmented into Bioburden Testing, Sterility Testing, and Bacterial Endotoxin Testing. Based on countries, the market is segmented into China, Japan, India, South Korea, Singapore, Malaysia, and Rest of Asia Pacific.
By Type
By Product Type
By Sample
By End Use
By Test Type
By Country
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

