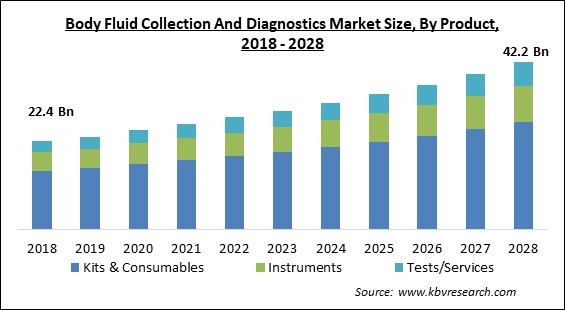
The Global Body Fluid Collection And Diagnostics Market size is expected to reach $42.2 billion by 2028, rising at a market growth of 6.9% CAGR during the forecast period.
The collection of body fluid and diagnosis requires various analytical specializations. Collection and diagnosis of body fluid consist of calculating and determining cells and additional particles. Calculating and determining cells from a range of various body fluids, for example, cerebrospinal fluid, serous fluids, and synovial fluid is achievable with urine flow cytometers and hematology analyzers.
The number and differentiation of cells in body fluids are essential parts of the process of discovering the essential diagnosis. There are different bases for asking this type of analysis, relying extremely on the type of body fluid. The presence of wide variety of pathological processes and diseases create an unusual accumulation of fluid in the body.
To find the fundamental cause of this, physicians mainly restrict and remove fluid burden, and send the fluid specimens to clinical laboratories for examination. By doing so, they provide an understanding of major points within the examination process of samples as part of the diagnostic and prognostic analysis. Labs utilize various diagnostic methods to test these samples, along with the measuring and detecting cells and biochemical evaluations and incubating process.
Gathering a blood sample and performing tests carefully are also major factors in the diagnosis of body fluid. Clinical laboratories are essential partners in offering operative outcomes for the patient’s management. But laboratories basically are not responsible for gathering body fluid similar to the phlebotomist collecting blood.
Furthermore, body fluid analysis is an off-label use of tests, mostly as per the vitro diagnostics manufacturers. According to laboratory accreditation and regulatory agencies, laboratories should do analytical validations for tests in which the type of sample identified is different from the purpose of use. These factors put lots of pressure on laboratories to identify and operate for pre-analytical conditions connected with body fluid collection and to prove the performance of any evaluation for which they claim to provide testing.
The COVID-19 pandemic has positively affected the body fluid collection and diagnosis market. This has raised the demand for body fluid diagnostic tests in pursuit of early identification of the disease. Blood, saliva, urine, and semen are collected from the patient to identify viral RNA in the specimen. Moreover, various enhancements have been made worldwide, which has also participated in the growth of the body fluid collection and diagnosis market. Because of the quick spread of the severe acute respiratory syndrome COVID-19, everybody is currently facing an uncommon situation worldwide. For this reason, the collection of body fluids became necessary at a wide scale, thereby supporting the market growth of body fluid collection and diagnostics.
As per the data provided by WHO, approximately 422 million people around the world have diabetes. The major proportion of patients are living in low-and-middle-income nations, and 1.5 million deaths are directly related to diabetes every year. The number of patients and the occurrence of diabetes have been constantly rising over the past few years. In 2014, 8.5 percent of adults of age 18 years and above had diabetes. In 2019, diabetes was the major reason for 1.5 million death and 48 percent of all deaths. 460,000 kidney disease deaths happened by diabetes, and increased blood glucose causes about 20 percent of cardiovascular deaths. This all has raised the growth opportunities to body fluid collection and diagnostics market.
There is a rise in the population of people aged 60 years and above. As per the data provided by the World Health Organization, in 2019, there were 1 billion people aged 60 years and older. This size will reach 1.4 billion by 2030 and 2.1 billion by 2050. This value is rising at an unmatched pace and will boost in the coming ten years. Specifically, in developing countries ageing brings significant burdens. It will rise the demand for basic health care and long-term care which need a more advanced and trained workforce. This will lead to the expansion of body fluid collection and diagnostics market.
As a result of the COVID-19 pandemic, laboratories and healthcare systems around the globe have been continuously under pressure. The lack of a workforce has made this worse. Because of the decline in the number of pathologists who are actively practicing, the issue of labor shortages becomes difficult to solve. Because of this severe absence of supply and demand balance, laboratories are required to work harder with fewer resources. Due to this, shortage of skilled workers, the market for body fluid collection and diagnostics may constrain as the alternative option may get more preference.
On the basis of the sample type, the body fluid collection and diagnostics market is classified into blood, saliva, urine, and cerebrospinal fluid. In 2021, the blood segment dominated the body fluid collection and diagnostics market with the maximum revenue share. The body fluid traces collected from crime scenes are essential to proof for forensic researchers. That body fluid has useful DNA proof, which can help in finding the suspect or victim. Crime scene exploration is the most important aspect of forensics.
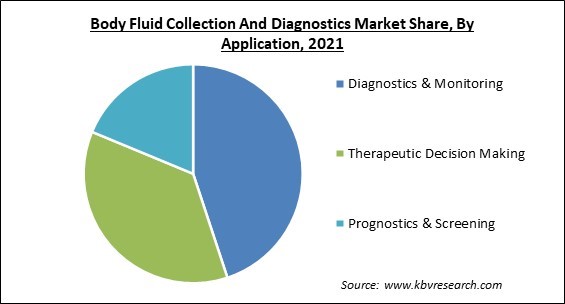
By application, the body fluid collection and diagnostics market is bifurcated into diagnostics & monitoring, therapeutic decision making and prognostics & screening. In 2021, therapeutic decision-making garnered a prominent growth rate in the body fluid collection and diagnostics market. The inclusion of the patient in therapeutic decision-making defines the advantages and dangers of treatment. This process helps physicians in concluding whether or not to provide treatment to patients who may have a disease or may not have the disease. This whole process is therapeutic decision-making.
On the basis of product, the body fluid collection and diagnostics market is fragmented into test/ services, kits & consumables, and instruments. In 2021, the kits & consumables segment witnessed the largest revenue share in the body fluid collection and diagnostics market. The rising demand for quick and affordable kits with supportive regulations by the government during the COVID-19 pandemic has participated in the rise of the market in this segment. For example, in 2021, COVID-19 home test kits got acceptance by medical researchers and concerned authorities who provided detailed information on how and who can use the kits.
Based on technology, the body fluid collection and diagnostic market is divided into next-generation sequencing, polymerase chain reaction, and fluorescence in situ hybridization. In 2021, the next generation sequencing segment garnered a substantial growth rate in the body fluid collection and diagnostics market. Next-generation sequencing has evolved in every field of biological science. It has tremendously decreased sequencing costs. Various enhancement has been happening in this field which is contributing to the growth of the body fluid collection and diagnostics market.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 26.7 Billion |
| Market size forecast in 2028 | USD 42.2 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 6.9% from 2022 to 2028 |
| Number of Pages | 296 |
| Number of Tables | 463 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Product, Technology, Sample Type, Application, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Region wise, the body fluid collection and diagnosis market is analyzed across North America, Europe, Asia Pacific and LAMEA. In 2021, North America region led the body fluid collection and diagnostics market by generating maximum revenue share. As per the data provided by the WHO, cardiovascular diseases are one of the major reasons for the death in North American region This acquires approximately 17.9 million death each year. More than four out of five CVD deaths are due to heart attacks, and one-third of these deaths happen prematurely in people under the age of 70. This increased burden of cardiovascular diseases would result in the rising need of body fluid collection & diagnostics, thus leading to market growth.
Free Valuable Insights: Global Body Fluid Collection And Diagnostics Market size to reach USD 42.2 Billion by 2028
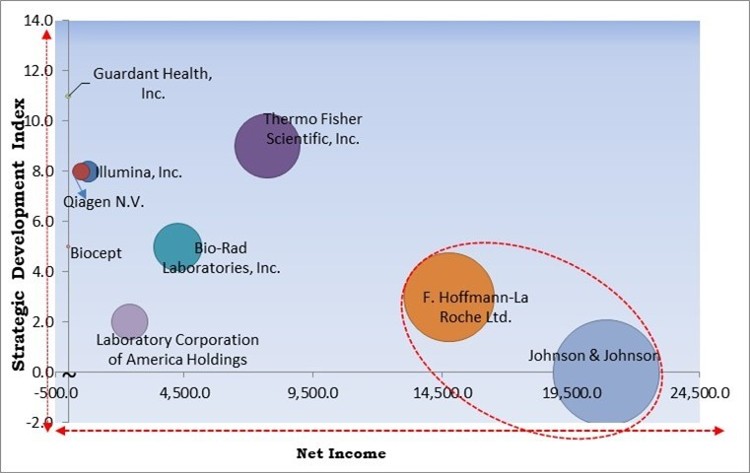
The major strategies followed by the market participants are Partnerships. Based on the Analysis presented in the Cardinal matrix; Johnson & Johnson and F. Hoffmann-La Roche Ltd. are the forerunners in the Body Fluid Collection And Diagnostics Market. Companies such as Thermo Fisher Scientific, Inc., Bio-Rad Laboratories, Inc., and Laboratory Corporation of America Holdings are some of the key innovators in Body Fluid Collection And Diagnostics Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Thermo Fisher Scientific, Inc., Bio-Rad Laboratories, Inc., Illumina, Inc., Qiagen N.V., Johnson & Johnson, F. Hoffmann-La Roche Ltd., Laboratory Corporation of America Holdings, Biocept, Inc., MDxHealth SA, and Guardant Health, Inc.
By Product
By Technology
By Sample Type
By Application
By Geography
The global Body Fluid Collection And Diagnostics Market size is expected to reach $42.2 billion by 2028.
Rising number of diabetic patients are driving the market in coming years, however, Unavailability of trained professionals restraints the growth of the market.
Thermo Fisher Scientific, Inc., Bio-Rad Laboratories, Inc., Illumina, Inc., Qiagen N.V., Johnson & Johnson, F. Hoffmann-La Roche Ltd., Laboratory Corporation of America Holdings, Biocept, Inc., MDxHealth SA, and Guardant Health, Inc.
The Polymerase Chain Reaction (PCR) segment acquired maximum revenue share in the Global Body Fluid Collection and Diagnostics Market by Technology in 2021 thereby, achieving a market value of $25 billion by 2028.
The Diagnostics & Monitoring segment is leading the Global Body Fluid Collection And Diagnostics Market by Application in 2021 thereby, achieving a market value of $18.4 billion by 2028.
The North America market dominated the Global Body Fluid Collection And Diagnostics Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $17.5 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

