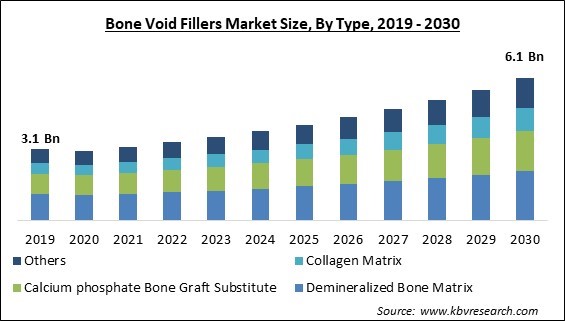
The Global Bone Void Fillers Market size is expected to reach $6.1 billion by 2030, rising at a market growth of 7.9% CAGR during the forecast period.
The main factor contributing to the rise of bone fracture, particularly in children, is a particular sport's year-round participation. Bone fracture is expected to acquire more than 53% share of the market by 2030. About 30 million children and teenagers in the United States take part in organized sports of some kind, and each year, these athletes suffer more than 3.5 million injuries that limit their ability to play for a period of time. Sports-related injuries account for nearly one-third of all injuries sustained by children. Sprains and strains are the most typical types of injuries. Undoubtedly, some sports are riskier than others. For instance, it is logical to anticipate that contact sports like football will cause more injuries than noncontact activities like swimming.
Sports-related injuries affect more than 775,000 kids aged 14 and under, who receive annual treatment in hospital emergency rooms. Sports-related injuries are therefore growing more frequent, especially in young people, which is expected to increase demand for bone void fillers. Some of the factors impacting the market are growing attention toward launching antibiotic eluting bone void fillers, rising acceptance of synthetic BVF, and lesser availability and clinical barriers.
Clinical restrictions, like bone infection after surgery, have heightened interest in using antibiotic-eluting void fillers. The market participants are focusing on accelerating R&D efforts and offering antibiotic-eluting void fillers that have also decreased bone infection while undergoing therapy. The introduction of antibiotic-eluting bone void fillers reflects the expanding demand for better infection control methods in orthopedic procedures. The launch of such cutting-edge products is projected to drive the market's expansion over the forecast period. Because they provide several advantages over conventional bone graft materials, synthetic bone void fillers (BVF) have increased usage in recent years. In place of autografts or allografts—which are taken from the patient's own body—synthetic BVFs are made-from-scratch materials. The market is predicted to develop due to the rising use of artificial bone void fillers such as calcium sulfate and tricalcium phosphate. The revenue generated was significantly lower in 2020 than it was in 2019. However, in 2021, elective procedures—including orthopedic & trauma surgery—began to resume as the government-imposed restrictions in various countries were removed. Due to several circumstances, including stringent requirements, implementing COVID-19 norms in public settings, and lifting travel restrictions, the number of patients and surgeries recovered. As a result, there was more demand for these products in 2021. Market growth prospects are anticipated to be consistent.
However, demand is declining due to various clinical limitations and inadequate bone void filling substance penetration. The major dangers and hazards associated with its usage, such as infection of soft tissue & bone, insufficient or non-existent bone growth, filler rupture, etc., cause this declining acceptance. Clinicians may be unsure of the best way to utilize and how these fillers will perform due to a lack of high-quality trials or a lack of long-term data. Therefore, these limitations are limiting the market's ability to grow.
Based on type, the market is segmented into demineralized bone matrix, calcium phosphate bone graft substitute, collagen matrix, and others. The calcium phosphate bone graft substitute segment acquired a substantial revenue share in the market in 2022. Bone void fillers frequently use calcium phosphate as a replacement for bone grafts. Using a bone void filler can fill in any gaps or defects in a bone, including those caused by bone cysts, fractures, or disease- or trauma-induced bone loss. Because of their biocompatibility and osteoconductive qualities, replacements made of calcium phosphate are frequently used for this purpose.
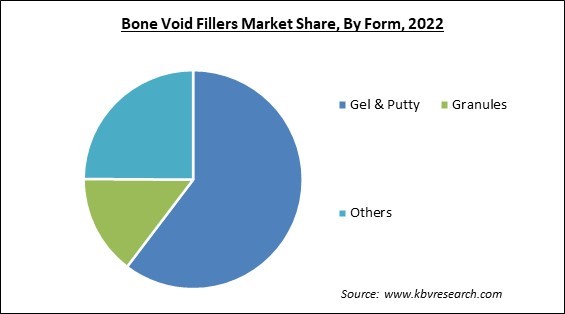
On the basis of form, the market is segmented into gel & putty, granules, and others. In 2022, the gel & putty segment witnessed the largest revenue share in the market. The segment's significant growth can be the result of the more non-invasive and minimally invasive operations being performed these days. Calcium phosphate-based substances, like hydroxyapatite (HA) or tricalcium phosphate (TCP) that enhance osteoconductivity and assist bone regeneration can be present in both gel & putty-based bone void fillers.
On the basis of application, the market is bifurcated into spine fusion, bone fracture and others. In 2022, the spine fusion covered a considerable segment revenue share in the market. To encourage the fusing of the spinal bones (vertebrae) and produce a solid and stable spinal column, bone void fillers are an essential component of spinal fusion surgeries. In a spine fusion, the choice of bone void fillers is influenced by several variables, including the specific surgical approach, the location & size of the bone void, the patient's health, and the surgeon's personal preferences. Based on the treatment's particular needs and the filler material's desired qualities, other types of bone graft substitutes, such as granules, putty, or gel-based fillers, may be employed.
By end user, the market is fragmented into hospital, specialty clinics, and others. In 2022, the hospital segment dominated the market with the maximum revenue share. The expansion of hospitals in both developed and developing nations is blamed for the growth. In addition, it is anticipated that during the anticipated timeframe, a sizable patient pool consulting hospitals to receive treatment and an increase in the number of orthopedic treatments performed in hospital facilities will support segmental expansion.
| Report Attribute | Details |
|---|---|
| Market size value in 2022 | USD 3.3 Billion |
| Market size forecast in 2030 | USD 6.1 Billion |
| Base Year | 2022 |
| Historical Period | 2019 to 2021 |
| Forecast Period | 2023 to 2030 |
| Revenue Growth Rate | CAGR of 7.9% from 2023 to 2030 |
| Number of Pages | 256 |
| Number of Table | 450 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Type, Form, Application, End User, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Region wise, the market is analysed across North America, Europe, Asia Pacific, and LAMEA. In 2022, the North America region led the market by generating the highest revenue share. This is attributable to the increased use of artificial bone void fillers, a sizable bone void filler business, the presence of a sophisticated healthcare system, and the widespread availability of bone void fillers.
Free Valuable Insights: Global Bone Void Fillers Market size to reach USD 6.1 Billion by 2030
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Medtronic PLC, DePuy Synthes (Johnson & Johnson), Orthofix Medical, Inc., Stryker Corporation, Zimmer Biomet Holdings, Inc., Smith & Nephew PLC, NuVasive, Inc., Baxter International, Inc., Biocomposites Ltd., and Arthrex, Inc.
By Type
By Form
By Application
By End User
By Geography
The Market size is projected to reach USD 6.1 billion by 2030.
Rising acceptance of synthetic BVF are driving the Market in coming years, however, Lesser availability and clinical barriers restraints the growth of the Market.
Medtronic PLC, DePuy Synthes (Johnson & Johnson), Orthofix Medical, Inc., Stryker Corporation, Zimmer Biomet Holdings, Inc., Smith & Nephew PLC, NuVasive, Inc., Baxter International, Inc., Biocomposites Ltd., and Arthrex, Inc.
The Demineralized Bone Matrix segment is generating the highest revenue share in the Global Bone Void Fillers Market by Type in 2022, achieving a market value of $2.1 billion by 2030.
The Bone Fracture segment is leading the Global Bone Void Fillers Market by Application in 2022, achieving a market value of $3.2 billion by 2030.
The North America market dominated the Global Bone Void Fillers Market by Region in 2022 and would continue to be a dominant market till 2030; thereby, achieving a market value of $2.5 billion by 2030.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

