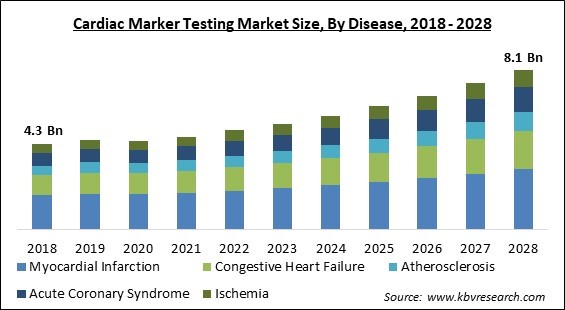
The Global Cardiac Marker Testing Market size is expected to reach $8.1 billion by 2028, rising at a market growth of 8.3% CAGR during the forecast period.
Protein molecules known as cardiac biomarkers are released into the blood following heart injury or heart stress. These biomarkers may be used to identify a number of cardiovascular disorders (CVD), such as acute coronary syndrome, cardiac ischemia, congestive heart failure, and myocardial infarction (ACS). The doctor can assess a patient's risk of contracting such conditions using cardiac biomarker tests.
Chemicals known as cardiac markers are released into the blood by the stressed or wounded heart. Acute coronary syndrome (ACS) and cardiac ischemia, disorders linked with insufficient blood supply to the heart, are diagnosed with the use of measurements of these biomarkers. Tests for cardiac biomarkers can also be employed to assess a person's likelihood of developing these diseases as well as to monitor and treat someone who has myocardial ischemia and probable ACS.
The development of plaque in artery walls and arterial hardening are typically the primary causes of both ACS as well as cardiac ischemia. This may lead to an abrupt obstruction of blood flow through these coronary arteries or a significant constriction of the arteries leading to the heart.
Troponin is the currently preferred biomarker test for identifying heart injury. In other circumstances, such as skeletal muscle injury, additional cardiac biomarkers may be raised and are less specific for the heart. The diagnosis, risk assessment, and treatment of patients with suspected acute coronary syndrome (ACS), acute heart failure, pulmonary embolism, and other disease states, as well as the prognosis for patients with these disorders, all rely on cardiac signs.
The cardiac markers testing market is anticipated to continue to benefit from the COVID-19 pandemic's effects. This is due to an increase in COVID-19 patients who have preexisting medical conditions, including cardiovascular illnesses and other conditions. Since there are more heart attacks in these patients with pre-existing medical disorders like cardiovascular disease, Troponin levels were also thought to be significantly greater in COVID-19 patients who passed away or were in critical condition. For patients with COVID-19, this has increased the demand for cardiac biomarker testing. Thus, taking into consideration all of these variables, the market for cardiac markers testing shows significant growth throughout this pandemic and is anticipated to increase further.
Numerous diseases and disorders are on the rise as people's lifestyles transform rapidly around the world. Lifestyle changes can lead to major health problems, including cardiovascular disease. An unhealthy diet, lack of physical activity, tobacco use, and excessive alcohol consumption are the primary modifiable risk factors for cardiovascular disease and stroke. Symptoms including hypertension, abnormal lipid profiles, diabetes, and obesity can all be attributed to a person's lifestyle choices.
One major factor increasing access to cardiac markers testing is the developing healthcare systems of various countries across the world. As a result of the increase in cancers diseases, and other disorders, governments around the world are investing more time and money into their healthcare systems. In addition, the healthcare industry is being pushed to adapt to new challenges by the ongoing pandemic, which continues to demand the attention and resources of healthcare systems around the world.
For the purpose of carrying out research into the various stages that diseases can progress through in humans, epidemiology studies make use of cardiac biomarkers. In order to extract a significant quantity of knowledge from a small number of biological samples, this method necessitates the careful processing and long-term storage of these samples. It is imperative that these samples undergo a thorough quality check in order to ensure the appropriate conditions for storage and prevent the loss of data.
Based on products, the cardiac marker testing market is segmented into reagents & kits and instruments. The instruments segment acquired a substantial revenue share in the cardiac marker testing market in 2021. To help identify the occurrence of ACS as well as cardiac ischemia and to assess their severity, cardiac biomarker tests are prescribed. A person with ACS or cardiac ischemia can be identified by rises in one or even more cardiac markers in the blood, enabling a quick and precise diagnosis and the right course of therapy.
On the basis of biomarker type, the cardiac marker testing market is fragmented into Troponin I & T, creatine kinase-MB(CK-MB), natriuretic peptide (Bnp Or Nt-Probnp), myoglobin, high-sensitivity C-reactive protein(hs-CRP), and other cardiac biomarkers. In 2021, the troponin I & T segment held the largest revenue share in the cardiac marker testing market. Due to its better sensitivity and specificity, cardiac troponin is the preferred biomarker again for the diagnosis of acute myocardial infarction. Recently, a number of high-sensitivity troponin tests have been introduced to the market.
By disease, the cardiac marker testing market is divided into myocardial infarction, congestive heart failure, acute coronary syndrome, atherosclerosis, and ischemia. In 2021, the acute coronary syndrome segment registered a substantial revenue share in the cardiac marker testing market. This is due to the rapidly increasing illness burden in poor and medium-income countries relative to high-income countries.
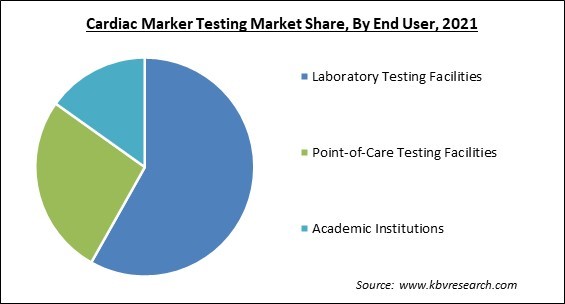
Based on end user, the cardiac marker testing market is classified into laboratory testing facilities, point of care testing facilities and academic institutions. In 2021, the laboratory testing segment generated the maximum revenue share in the cardiac marker testing market. This growth is attributable to factors such as the reform in healthcare infrastructure, the rise in healthcare spending, the rise in spending power, and initiatives for preventing heart attacks.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 4.7 Billion |
| Market size forecast in 2028 | USD 8.1 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 8.3% from 2022 to 2028 |
| Number of Pages | 303 |
| Number of Tables | 483 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Disease, Product, Biomarker Type, End User, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Region wise, the cardiac marker testing market is analyzed across North America, Europe, Asia Pacific and LAMEA. In 2021, the North America region led the cardiac marker testing market with the highest revenue share. Several factors contribute to this which include, substantial investment in research and development, the presence of key players and ready access to their products, and a mature healthcare system in the region.
Free Valuable Insights: Global Cardiac Marker Testing Market size to reach USD 8.1 Billion by 2028
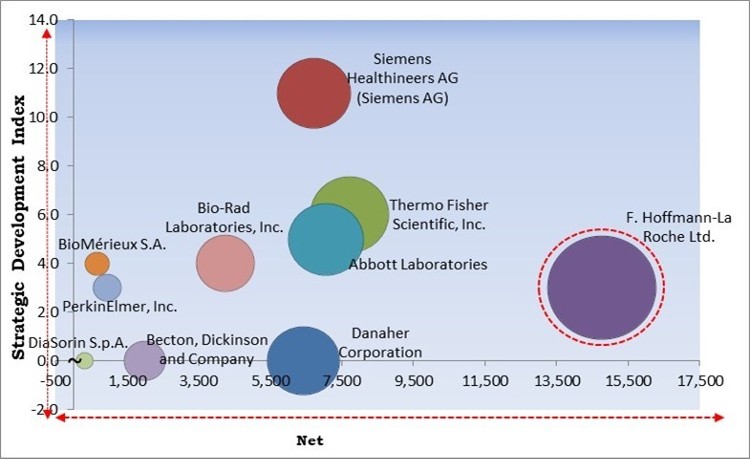
The major strategies followed by the market participants are Product Launches. Based on the Analysis presented in the Cardinal matrix; F. Hoffmann-La Roche Ltd. is the major forerunner in the Cardiac Marker Testing Market. Companies such as Abbott Laboratories, Thermo Fisher Scientific, Inc. and Danaher Corporation are some of the key innovators in Cardiac Marker Testing Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include F. Hoffmann-La Roche Ltd., Abbott Laboratories, Danaher Corporation, Siemens Healthineers AG (Siemens AG), PerkinElmer, Inc., BioMérieux S.A., Becton, Dickinson and Company, Bio-Rad Laboratories, Inc., Thermo Fisher Scientific, Inc., and DiaSorin S.p.A.
By Disease
By End User
By Product
By Biomarker Type
By Geography
The global Cardiac Marker Testing Market size is expected to reach $8.1 billion by 2028.
Rising Prevalence Of Cardiovascular Diseases are driving the market in coming years, however, Technical Issues With Sample Storage & Collecting restraints the growth of the market.
F. Hoffmann-La Roche Ltd., Abbott Laboratories, Danaher Corporation, Siemens Healthineers AG (Siemens AG), PerkinElmer, Inc., BioMérieux S.A., Becton, Dickinson and Company, Bio-Rad Laboratories, Inc., Thermo Fisher Scientific, Inc., and DiaSorin S.p.A.
The Myocardial Infarction segment is generating highest revenue share in the Global Cardiac Marker Testing Market by Disease in 2021 thereby, achieving a market value of $3.1 billion by 2028.
The Reagents & Kits segment is leading the Global Cardiac Marker Testing Market by Product in 2021 thereby, achieving a market value of $5.1 billion by 2028.
The North America market dominated the Global Cardiac Marker Testing Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $3 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

