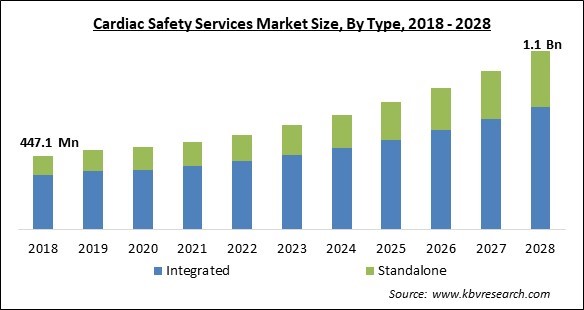
The Global Cardiac Safety Services Market size is expected to reach $1.1 billion by 2028, rising at a market growth of 11.1% CAGR during the forecast period.
Cardiac safety services assist and design clinical studies & trials and other research necessary for the monitoring of heart safety. These are primarily concerned with tracking the full cardiac safety profile through all clinical trial phases, from Phase I through Phase IV. Guidelines and regulations given by most major and recognized medical authorities of various countries and other regulating agencies are followed while developing cardiac safety clinical trials and studies.
These services include physiologic stress testing, non-invasive cardiac imaging, platelet aggregation, ambulatory blood pressure monitoring, and other services in addition to the QT tests. Due to the grouping of services with an emphasis on end-to-end development, integrated services that provide cardiac safety services are generally favored over standalone services because of the peculiarity of standalone services.
All elements of the cardiovascular system, including the heart, blood vessels, and blood constituents, are affected by cardiovascular safety liabilities, which include both cardiovascular and non-cardiovascular pharmaceuticals. Cardiovascular adverse effects can be functional or structural (such as histopathology) in nature and can happen after acute or chronic treatment.
The need for effective yet secure drug development is more critical than ever in a time of growing public scrutiny, rising business expenses, and limited resources at regulatory agencies. With 17.9 million deaths per year, cardiovascular diseases (CVDs) are the most common cause of death worldwide. Cerebrovascular disease, coronary heart disease, rheumatic heart disease, and other illnesses are among the category of heart and blood vessel disorders known as CVDs.
This prompted the pharmaceutical and health insurance industries to make the necessary changes to their offerings in order to include better provisions and facilities. In order to progress the evolution of medicine, this compelled the engagement of numerous research institutions and organizations dedicated to heart health. Coronavirus-related catastrophes caused a significant number of patients with pre-existing unfavorable disorders to succumb to them, which had an effect on cardiac safety services. As a result of COVID-19's vigorous promotion of its development, the market for cardiac safety services was positively affected overall.
Numerous businesses are making significant investments in the creation of biologics and biosimilar compounds. In the discovery stage, biologics such peptides, proteins, and monoclonal antibodies make up more than half of the therapeutic candidates. Pharmaceutical and biopharmaceutical businesses are heavily putting money into their research and development as novel biologics are being developed or are in the pipeline. Biosimilars are also less expensive because, being generic versions of patented biologic medications, they are not subject to the same strict regulatory standards.
The rise in the number of elderly people and the increasing incidence of cardiovascular disorders are mostly to blame for the ECG Holter's expansion. According to data released by Health System Tracker, heart disease is the top cause of mortality in the United States. These reasons make immediate heartbeat detection and constant heart rate monitoring equipment necessary. In the past few years, the healthcare system has employed ECG monitoring devices to look for any abnormalities in cardiac activity.
The R&D outsourcing industry for pharmaceuticals, biotechnology, and medical devices is always changing. To deliver high-quality services, adhere to acceptable laboratory procedures, and keep up with the ongoing developments in medical device and pharmaceutical R&D technologies and techniques, highly skilled experts are needed. As they compete for trained and experienced scientists with biotechnology, pharmaceutical, and medical device businesses as well as research and academic organizations, CROs confront difficulties in attracting and keeping highly skilled personnel.
Based on type, the cardiac safety services market is bifurcated into integrated and standalone. The integrated segment dominated the cardiac safety services market with the highest revenue share in 2021. These cutting-edge core lab services provide a full range of cardiac safety services, including imaging, TQT, and profile QT tests. In order to help with the real-time evaluation of heart rate and rhythm, they also keep an eye on off-target cardiovascular liabilities and conduct onsite multichannel telemetry under the supervision of licensed nurses.
On the basis of type of service, the cardiac safety services market is divided into ECG/Holter measurement, blood pressure measurement, cardiovascular imaging, thorough QT studies, and other services. The ECG/Holter measurement segment witnessed the largest revenue share in the cardiac safety services market in 2021. Smartwatches and other personal electronics provide electrocardiogram monitoring. A small, portable gadget called a Holter monitor is used to capture heartbeats. It is employed to identify and evaluate the likelihood of aberrant heartbeats (arrhythmias).
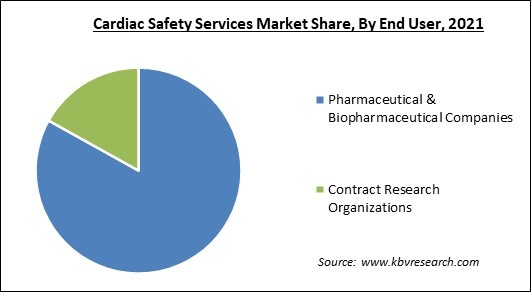
Based on end user, the cardiac safety services market is categorized into pharmaceutical & biopharmaceutical companies and contract research organizations. The contract research organizations segment procured a substantial revenue share in the cardiac safety services market in 2021. Clinical trials are carried out by Contract Research Organizations (CROs) for the pharmaceutical, medical device, and biotechnology businesses as well as for academic institutions, governmental agencies, and foundations.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 528.1 Million |
| Market size forecast in 2028 | USD 1.1 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 11.1% from 2022 to 2028 |
| Number of Pages | 212 |
| Number of Tables | 354 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Type, Type of Service, End User, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
On the basis of region, the cardiac safety devices is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America region acquired the highest revenue share in the cardiac safety services market in 2021. This is due to the region's growing number of clinical studies, which are fueling demands for cardiac safety services. The rising number of people suffering from cardiovascular diseases (CVD) and coronary heart disease (CHD) have accelerated the drug development and research studies in the region.
Free Valuable Insights: Global Cardiac Safety Services Market size to reach USD 1.1 Billion by 2028
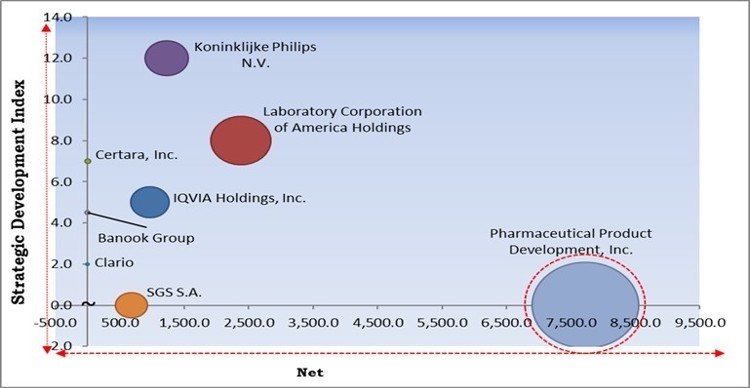
The major strategies followed by the market participants are Acquisitions. Based on the Analysis presented in the Cardinal matrix; Pharmaceutical Product Development, Inc. is the major forerunner in the Cardiac Safety Services Market. Companies such as Koninklijke Philips N.V., Laboratory Corporation of America Holdings, IQVIA Holdings, Inc. are some of the key innovators in Cardiac Safety Services Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Koninklijke Philips N.V., Clario, IQVIA Holdings, Inc., Laboratory Corporation of America Holdings, Pharmaceutical Product Development, Inc., SGS S.A., Banook Group, Biotrial Research SAS, Certara, Inc., and Celerion.
By Type
By End User
By Type of Service
By Geography
The global Cardiac Safety Services Market size is expected to reach $1.1 billion by 2028.
Increase In Research For Biosimilars And Biologics are driving the market in coming years, however, Lack Of Qualified Personnel For Clinical Trials restraints the growth of the market.
Koninklijke Philips N.V., Clario, IQVIA Holdings, Inc., Laboratory Corporation of America Holdings, Pharmaceutical Product Development, Inc., SGS S.A., Banook Group, Biotrial Research SAS, Certara, Inc., and Celerion.
The Pharmaceutical & Biopharmaceutical Companies segment is leading the Global Cardiac Safety Services Market by End User in 2021 thereby, achieving a market value of $864.1 Million by 2028.
The North America market dominated the Global Cardiac Safety Services Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $403.1 Million by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

