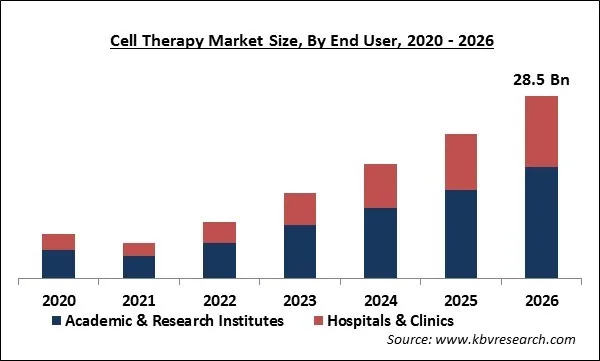
The Global Cell Therapy Market size is expected to reach $28.5 billion by 2026, rising at a market growth of 26.2% CAGR during the forecast period. Cell therapy is considered as a technology that is based on substituting dysfunctional or diseased cells with active & functional cells. Stem cells possess the capability to differentiate into particular cells needed for repairing damaged or defective tissues or cells, that’s why they are utilized for these advanced therapies. In addition, cell therapy is used in the development of regenerative medicines, which is an interdisciplinary domain aimed at maintenance, enhancement, or restoration of cell, tissue, organ function with the help of procedures majorly associated with cell therapy.
Moreover, cells like bone marrow and blood cells, mature, immature & solid tissue cells, adult stem cells, and embryonic cells are extensively deployed in cell therapy methods. Further, transplanted cells involve embryonic stem cells (ESCs), pluripotent stem cells (iPSCs), neural stem cells (NSCs), and mesenchymal stem cells (MSCs) are bifurcated mainly two groups viz. Allogeneic cell therapy and non-Allogeneic cell therapy.
At present, there are two types of cell therapies exist. The first type is cell therapy in mainstream medicine. This category includes human embryonic stem cell therapy, mesenchymal stem cell therapy, neural stem cell therapy, Autologous cell therapy, and hematopoietic stem cell therapy. The second category is in alternative medicine and perpetuates the practice of injecting animal materials in an effort to cure diseases. This practice, as per the American Cancer Society, is not supported by any medical proof of efficiency and can have fatal results.
The outbreak of the COVID-19 pandemic has affected many biopharmaceutical units, while many cellular therapy development organizations have observed a severe impact, which is due to the complexities in logistics and manufacturing models used in this sector. Moreover, considerable and constant investment is essential to allow effective commercial translation of cell-based therapeutics, an aspect that was negatively affected in 2020, further affected the overall market growth.
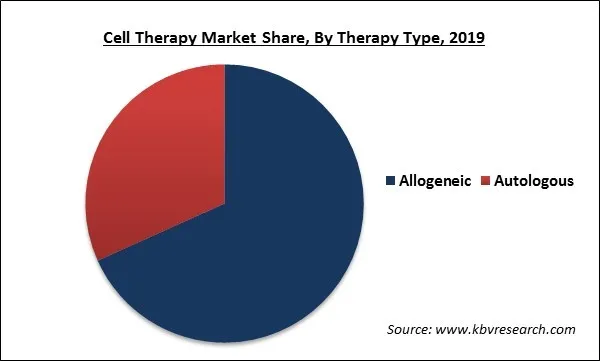
Based on Therapy Type, the market is segmented into Autologous and Allogeneic. The Autologous therapies segment would show gradual growth due to the massive cost associated with Autologous transplants and comparatively less relapse rates. Though, development in cell banking and companies’ switch towards the creation of allogenic therapy products is boosting the cell therapy market.
Based on Therapeutic Area, the market is segmented into Malignancies, Autoimmune Disorders, Musculoskeletal Disorders, Dermatology and Others. The Malignancies market dominated the Global Cell Therapy Market by Therapeutic Area 2019, growing at a CAGR of 25.2 % during the forecast period. The Autoimmune Disorders market is experiencing a CAGR of 27.6% during (2020 - 2026). Additionally, The Musculoskeletal Disorders market would showcase a CAGR of 27.2% during (2020 - 2026).
Based on End User, the market is segmented into Academic & Research Institutes and Hospitals & Clinics. In 2019, the Academic & Research Institutes segment emerged as the leading segment of the market and held the highest revenue share. The stem cells are significantly being utilized for research projects, which as a result, has contributed to a massive revenue share for the Academic & Research Institutes segment. The segment would show a similar trend during the forecast period.
Based on Cell Type, the market is segmented into Stem Cell, Umbilical Cord-Derived, Adipose-Derived Stem Cell, Non-Stem Cell, Bone Marrow, Blood and Others. The Stem Cell market dominated the Global Cell Therapy Market by Cell Type 2019, and would continue to be a dominant market till 2026. The Umbilical Cord-Derived market is expected to witness a CAGR of 27.7% during (2020 - 2026).
| Report Attribute | Details |
|---|---|
| Market size value in 2019 | USD 5.8 Billion |
| Market size forecast in 2026 | USD 28.5 Billion |
| Base Year | 2019 |
| Historical Period | 2016 to 2018 |
| Forecast Period | 2020 to 2026 |
| Revenue Growth Rate | CAGR of 26.2% from 2020 to 2026 |
| Number of Pages | 270 |
| Number of Tables | 485 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling, Competitive Landscape |
| Segments covered | Therapy Type, Therapeutic Area, End User, Cell Type, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Free Valuable Insights: Global Cell Therapy Market to reach a market size of $28.5 Billion by 2026
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. In 2019, North America emerged as the leading region of the global cell therapy market by obtaining the highest revenue share. The larger share of the region is due to the existence of a considerable number of centers and institutes that are undergoing R&D activities to stem cell therapy. Among the top universities in the world, 5 are based in the U.S., including Institute for Stem Cell Biology and Regenerative Medicine, Stanford University, Harvard Stem Cell Institute, Harvard University, and Yale Stem Cell Center.
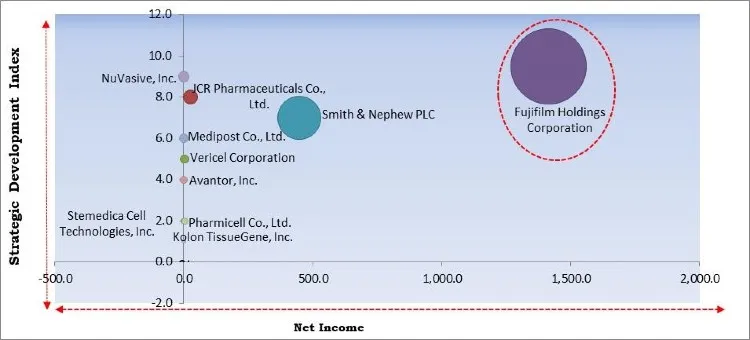
The major strategies followed by the market participants are Partnerships. Based on the Analysis presented in the Cardinal matrix; Fujifilm Holdings Corporation is the major forerunner in the Cell Therapy Market. Companies such as JCR Pharmaceuticals Co., Ltd., NuVasive, Inc., Medipost Co., Ltd., Vericel Corporation, and Avantor, Inc. are some of the key innovators in the market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Fujifilm Holdings Corporation, Smith & Nephew PLC, Kolon TissueGene, Inc., JCR Pharmaceuticals Co., Ltd., Medipost Co., Ltd., Stemedica Cell Technologies, Inc., NuVasive, Inc., Avantor, Inc., Vericel Corporation, and Pharmicell Co., Ltd.
By Therapy Type
By Therapeutic Area
By End User
By Cell Type
By Geography
The global cell therapy market size is expected to reach $28.5 billion by 2026.
Increasing government funding in cell-based research are driving the market in coming years, however, lack of skilled professionals have limited the growth of the market.
The outbreak of the COVID-19 pandemic has affected many biopharmaceutical units, while many cellular therapy development organizations have observed a severe impact, which is due to the complexities in logistics and manufacturing models used in this sector.
Fujifilm Holdings Corporation, Smith & Nephew PLC, Kolon TissueGene, Inc., JCR Pharmaceuticals Co., Ltd., Medipost Co., Ltd., Stemedica Cell Technologies, Inc., NuVasive, Inc., Avantor, Inc., Vericel Corporation, and Pharmicell Co., Ltd.
The expected CAGR of the cell therapy market is 26.2% from 2020 to 2026.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

