
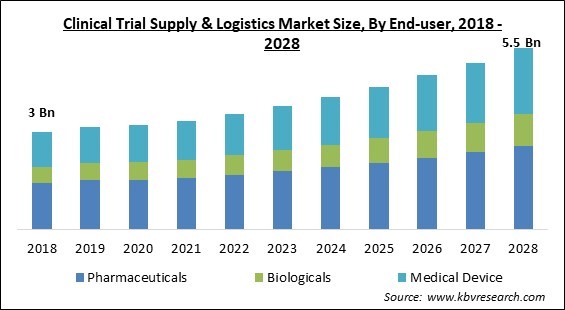
The Global Clinical Trial Supply & Logistics Market size is expected to reach $5.5 billion by 2028, rising at a market growth of 7.8% CAGR during the forecast period.
Clinical trial supply and logistics entail managing clinical supplies required for clinical investigations of pharmaceuticals or medical devices in line with protocol and applicable regulatory standards. This procedure oversees the planning, packaging, forecasting, labeling, sourcing, and distribution of clinical goods for commercial and government sponsors whose clinical trials are in phases 1-4.
Clinical trial providers collaborate with numerous third-party vendors and technicians at various stages of clinical distribution to confirm that the study medications offered are of sufficient amount and quality. This management, in terms of medicine supply, provides full tracking from manufacturing to dispensing and disposal, reducing risks such as product expiration or depletion.
The increased number of clinical trials and harmonization of regulations, rising R&D spending by pharmaceutical and biotechnology companies, and technical developments in the supply chain are major market drivers. Due to the increase in the expense of clinical trials, there is also an increase in outsourcing these services.
The COVID-19 pandemic imposed logistical obstacles on the clinical trial industry and sponsors encountered numerous challenges during the pandemic. The rapid and widespread adoption of the remote trial technique has significantly altered the traditional supply chain. Direct-to-patient solutions were utilized to address the diverse logistical challenges of remote research. Decentralized trials facilitate sponsors' access to a broad audience. Even though the pandemic has boosted the acceptance of these trials, there will be a significant demand for them even after COVID-19 is eradicated.
COVID-19 negatively influenced the market due to disruptions in the supply chain and the government's decision to reduce the number of commercial airlines to limit the spread of the virus. However, during the second half of the pandemic, the market began to recover due to increased demand for COVID- 19 vaccinations. According to the WHO, there are 169 vaccines in the clinical development phase and 198 in the preclinical phase as of August 2, 2022. Due to the rising requirement for effective logistics and supply chain management, which comprises temperature control management and cold chain management, clinical trials are increasingly outsourced.
In recent years, the number of registered clinical trials has expanded dramatically. As of January 22, 2023, around 4,39,527 reported clinical studies were reported. Recent years have seen an increase in the complexity of clinical trials, which remain indispensable for the R&D of novel medications and technologies. The pharmaceutical industry spends the highest proportion of its income on research and development compared to other sectors. According to data, the number of registered and active clinical trial cases has steadily increased over the past decade. This has increased outsourcing-related tasks, such as material supply, blinding, delivery, and packaging. Consequently, these factors drive the expansion of the Clinical Trial Supply & Logistics Market.
Regarding trial expenses and patient pools, North America and Europe, the traditional centers of clinical trials, are confronting difficulties. This has increased the incidence of uncommon diseases, and their clinical trials are being relocated to poorer countries. Moreover, technology and data play a leading role in clinical trial decision-making. Catalent, one of the top competitors in this market, invested USD 9 million in a new clinical supply center in San Diego to provide comprehensive clinical supply services for early-phase clinical studies. In India, Biocair teamed with Linehaul Express to expand its presence in new markets. Marken (a UPS subsidiary) acquired PCX International in Japan to strengthen its presence in the local market.
The high expense of clinical trials is a significant barrier to expanding the market globally. The escalating cost of clinical trials is an essential cause for concern. The increasing requirement to collect more clinical data is a factor that contributes to the rising expenses of clinical studies. The researchers overseeing a clinical study must consider trial design, site selection, protocol design, and trial execution when preparing the trial. The National Health Service (NHS) does not fund clinical trial research in the United Kingdom. Drug firms, charitable organizations, government-funded bodies like the Medical Research Council (MRC) and the National Institute of Health Research (NIHR), and occasionally foreign organizations provide funding. All of these factors restrain the expansion of the Clinical Trial Supply & Logistics Market over the forecast period.
Based on service, the clinical trial supply & logistics market is segmented into logistics & distribution, storage & retention, packaging, labeling, & blinding, manufacturing, comparator sourcing, and other services. The manufacturing segment acquired a significant revenue share in the clinical trial supply & logistics market in 2021. This is a result of the increasing demand for biologics and complicated molecules, the expansion of manufacturing facilities, and the rise in clinical trials, which has led to an increase in material supply and the need for quality medication manufacturing.
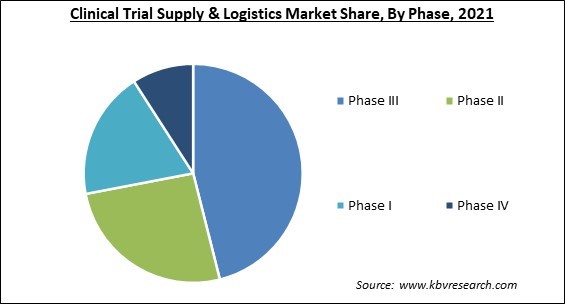
Based on phase, the clinical trial supply & logistics market is segmented into phase I, phase II, phase III, and phase IV. The phase II segment procured a substantial revenue share in the clinical trial supply & logistics market in 2021. This is due to the increase in outsourcing in this phase and an increase in investments by industry and non-industry sponsors. The increasing number of medications in phase II is anticipated to cause more significant complications in logistics and supply chain, increasing the demand for efficient logistics and supply chain, consequently contributing to the segment growth.
Based on end-user, the clinical trial supply & logistics market is segmented into pharmaceuticals, biologicals, and medical device. The biologicals segment garnered a prominent revenue share in the clinical trial supply & logistics market in 2021. Increasing adoption of biologics products like vaccines, cell and gene therapies, and investments for product manufacture and development are the primary market drivers. In addition, the desire for vaccination globally to avert the COVID-19 pandemic is increasing. The increasing adoption coupled with other features is expected to surge the segment’s growth.
Based on therapeutic area, the clinical trial supply & logistics market is segmented into oncology, cardiovascular diseases, respiratory diseases, CNS & mental disorders, and others. The oncology segment recorded a promising growth rate in the clinical trial supply & logistics market in 2021. This is because of the highly sophisticated therapeutic approaches, trials, and ever-changing demand for supportive services. Oncology clinical trials aim to diagnose, monitor, and treat cancer and its accompanying symptoms. Also, the oncology clinical trial supplies use both primary and secondary packing. The fundamental goal of packaging is to increase patient adherence. The above-mentioned characteristics are anticipated to surge the segment’s growth.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 3.3 Billion |
| Market size forecast in 2028 | USD 5.5 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 7.8% from 2022 to 2028 |
| Number of Pages | 249 |
| Number of Tables | 500 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Therapeutic Area, Phase, Service, End-user, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Based on region, the clinical trial supply & logistics market is segmented into North America, Europe, Asia Pacific, and LAMEA. In 2021, the North America region generated the highest revenue share in the clinical trial supply & logistics market. Regionally, the most significant number of clinical trials are undertaken which is a primary growth driver for the market. The substantial increase in spending in clinical trials and the rise in market participants are anticipated to contribute to market expansion in the North America region during the projected period.
Free Valuable Insights: Global Clinical Trial Supply & Logistics Market size to reach USD 5.5 Billion by 2028
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Thermo Fisher Scientific, Inc., Catalent, Inc., Marken (United Parcel Service of America, Inc.), Piramal Enterprises Limited, FedEx Corporation, Inizio Group Limited (Clayton, Dubilier & Rice), Parexel International Corporation (Phoenix Parentco, Inc.), Almac Group, Movianto (Walden Group SAS), and Deutsche Post DHL Group (The Deutsche Post AG)
By End-user
By Therapeutic Area
By Phase
By Service
By Geography
The global Clinical Trial Supply & Logistics Market size is expected to reach $5.5 billion by 2028.
A growing number of clinical trials registered are driving the market in coming years, however, Expensive clinical trials restraints the growth of the market.
Thermo Fisher Scientific, Inc., Catalent, Inc., Marken (United Parcel Service of America, Inc.), Piramal Enterprises Limited, FedEx Corporation, Inizio Group Limited (Clayton, Dubilier & Rice), Parexel International Corporation (Phoenix Parentco, Inc.), Almac Group, Movianto (Walden Group SAS), and Deutsche Post DHL Group (The Deutsche Post AG)
The expected CAGR of the Clinical Trial Supply & Logistics Market is 7.8% from 2022 to 2028.
The Pharmaceuticals segment acquired maximum revenue share in the Global Clinical Trial Supply & Logistics Market by End-user in 2021 thereby, achieving a market value of $2.5 billion by 2028.
The North America market dominated the Global Clinical Trial Supply & Logistics Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $2.1 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
