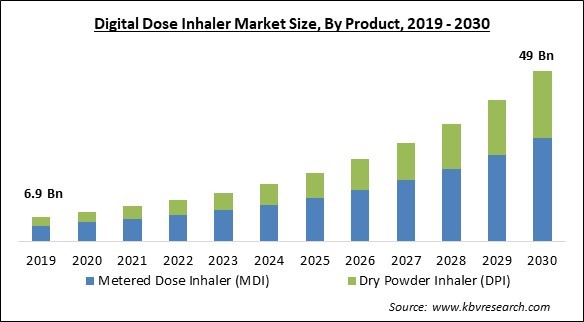
The Global Digital Dose Inhaler Market size is expected to reach $49 billion by 2030, rising at a market growth of 19.6% CAGR during the forecast period.
Cigarette smoking and long-term exposure to other respiratory contaminants are typically the cause of various diseases, including asthma. The asthma segment will capture more than 30% share of the market by 2030. The World Health Organization (WHO) estimates that annually, tobacco kills more than eight million people. 7 million deaths are due to direct tobacco use, while approximately 1.2 million are due to latent or secondhand smoking. Cigarette smoking is one of the most prevalent forms of tobacco use worldwide. Over 80% of the 1.3 billion tobacco consumers reside in low- and middle-income countries, where mortality and illness due to tobacco use are prevalent. Some of the factors impacting the market are globally increasing air pollution, developing technological advances and concerns related to patient data security.
Automobiles, forest fires, household combustion, and industrial facilities are widely recognized sources of air pollution. Particulate matter, ozone, carbon monoxide, sulfur dioxide, and nitrogen are the pollutants of most threat to public health. Both indoor and external air pollution contribute significantly to mortality and morbidity by causing respiratory and other diseases. According to the World Health Organization, nearly all the world's population consumes air with elevated levels of pollutants exceeding WHO guidelines. Low- and middle-income countries are the most vulnerable to these threats. Additionally, the increasing adoption of AI and IoT has been identified as one of the most significant market trends. AI technology analyzes intricate medical data using algorithms and software, reducing the need for direct human input. This will aid in decreasing the likelihood of human error, reducing treatment costs, and enhancing healthcare outcomes. Therefore, Long-term exposure to ambient air contaminants is associated with an increase in the incidence of respiratory diseases and the increasing incorporation of AI and the adoption of IoT will improve the treatment of asthma and COPD, thereby increasing demand.
Furthermore, in order to provide care for the infected population, there is a growing demand for medical supplies. Respiratory support devices like atomizers, oxygen generators, life-support machines, and monitors are some of the most frequently utilized medical devices in primary medical care. The continuous outbreak of the COVID-19 pandemic had a positive effect on the medical device industry. It affects the market for digital dose inhalation. However, this escalating pandemic affects the supply chain and reduces demand for digital dose inhalation devices, resulting in the temporary shutdown of industries and the announcement of lockdowns by governments in numerous regions.
Additionally, due to concerns about patient data security, it is predicted that the market will expand slowly. Due to a lack of knowledge of the symptoms of COPD and asthma, the vast majority of individuals with respiratory illnesses go undiagnosed and are consequently given the incorrect treatment. This may be a result of patients not being technologically savvy and the payment restrictions. The device's limited availability is another issue that, given the existing circumstance, will have a negative impact on the market for digital dosage inhalers throughout the projection period.
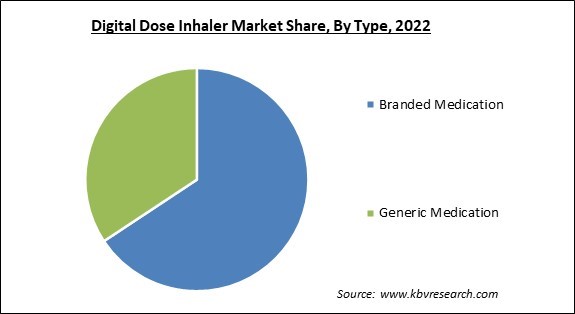
On the basis of type, the market is fragmented into branded medication and generic medication. The generic medication segment garnered the highest revenue share in the market. Frequently, digital inhalers include sensors and connectivity features that monitor and track medication usage. They can log the date, time, and frequency of inhaler use, providing valuable information to patients, healthcare providers, and researchers. A few digital inhalers can be programmed with medication reminders and alerts.
Based on Product, the market is segmented into metered dose inhaler (MDI) and dry powder inhaler (DPI). In 2022, the metered dose inhaler (MDI) segment held the highest revenue share in the market. This is due to rising healthcare costs and increased respiratory disease cases. Furthermore, consistent technological advancements related to the development of inhalation medication, like mechanistic Pharmacokinetic/Pharmacodynamics (PK/PD) modeling, and nanotechnology-based formulations with high drug loads delivered in smaller dose sizes, are some of the critical factors involved in enhancing the therapeutic efficacy of the medication and influencing the overall market.
By indication, the market is bifurcated into asthma, chronic obstructive pulmonary disease (COPD), and others. In 2022, the chronic obstructive pulmonary disease (COPD) segment dominated the market. The segment is anticipated to dominate over the forecast period due to the global increase in COPD incidence. Tobacco use, air pollution, pollen, chemical vapors, and childhood infections are all causes of COPD. Digital Dose Inhalers aid COPD patients in monitoring their respiratory health and drug adherence, enabling them to manage their illness more effectively.
| Report Attribute | Details |
|---|---|
| Market size value in 2022 | USD 11.8 Billion |
| Market size forecast in 2030 | USD 49 Billion |
| Base Year | 2022 |
| Historical Period | 2019 to 2021 |
| Forecast Period | 2023 to 2030 |
| Revenue Growth Rate | CAGR of 19.6% from 2023 to 2030 |
| Number of Pages | 230 |
| Number of Table | 330 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Product, Type, Indication, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. In 2022, the North America region led the market by generating the highest revenue share. This is due to rigorous research and development efforts, as well as an increased awareness of the high-tech respiratory devices currently available. In addition, an increase in the prevalence of respiratory conditions among children and older people will drive market expansion over the forecast period.
Free Valuable Insights: Global Digital Dose Inhaler Market size to reach USD 49 Billion by 2030
The market research report covers the analysis of key stakeholders of the market. Key companies profiled in the report include Sensirion AG, Teva Pharmaceuticals Industries Ltd., OPKO Health, Inc., Propeller Health (ResMed, Inc.), Novartis AG, AstraZeneca PLC, Glenmark Pharmaceuticals Limited, Beximco Pharmaceuticals Ltd., GlaxoSmithKline PLC and Mundipharma Deutschland GmbH & Co. KG.
By Product
By Type
By Indication
By Geography
The Market size is projected to reach USD 49 billion by 2030.
Developing technological advances are driving the Market in coming years, however, Concerns related to patient data security restraints the growth of the Market.
Sensirion AG, Teva Pharmaceuticals Industries Ltd., OPKO Health, Inc., Propeller Health (ResMed, Inc.), Novartis AG, AstraZeneca PLC, Glenmark Pharmaceuticals Limited, Beximco Pharmaceuticals Ltd., GlaxoSmithKline PLC and Mundipharma Deutschland GmbH & Co. KG.
The Branded Medication segment is leading the Market by Type in 2022; thereby, achieving a market value of $30.3 billion by 2030.
The North America region dominated the Market by Region in 2022, and would continue to be a dominant market till 2030; thereby, achieving a market value of $20.4 billion by 2030.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

