The Europe Life Sciences Quality Management Software Market would witness market growth of 12.2% CAGR during the forecast period (2024-2031).
The Germany market dominated the Europe Life Sciences Quality Management Software Market by Country in 2023, and would continue to be a dominant market till 2031; thereby, achieving a market value of $482.6 million by 2031. The UK market is exhibiting a CAGR of 11.3% during (2024 - 2031). Additionally, The France market would experience a CAGR of 13% during (2024 - 2031).
Audits are essential for ensuring compliance with regulatory requirements. Life sciences QMS software helps streamline the audit process by providing tools for scheduling, tracking, and managing internal and external audits. The software enables real-time audit reporting and non-conformance tracking, which helps organizations address issues swiftly and mitigate the risk of regulatory violations.
Risk management is a critical quality assurance component in the life sciences industry. Life sciences QMS software allows organizations to identify, assess, and mitigate product quality, safety, and compliance risks. This can include everything from identifying potential hazards in manufacturing processes to assessing the risks of regulatory non-compliance.
The high volume of biotech patents, especially in the medical and industrial domains, indicates a surge in product development, clinical trials, and manufacturing activities. These activities necessitate reliable QMS systems to manage quality, documentation, and compliance in an increasingly complex and competitive environment. As European companies lead in biotechnology innovation, they will continue to face growing pressure to maintain high standards in product development, regulatory adherence, and safety, creating a significant opportunity for Life Sciences QMS providers to cater to this market. The increasing sophistication of biotech operations in Europe ensures a continued demand for software solutions that streamline operations and ensure the highest quality and compliance standards. Thus, the rising biotech sector in Europe is propelling the market's growth.
Free Valuable Insights: The Global Life Sciences Quality Management Software Market will Hit USD 7.17 Billion by 2031, at a CAGR of 12.6%
Based on Application, the market is segmented into Data Management, Regulatory & Compliance Management, Corrective Action Preventive Action (CAPA) Management, Change Management, Audit Management, Risk Management, Non-Conformances Management, Supplier Management, Training Management, Inspection Management, and Other Application. Based on Deployment Mode, the market is segmented into Cloud & Web-based and On-premises. Based on End Use, the market is segmented into Pharmaceutical Firms, Biotech Firms, and CROs/CDMOs. Based on countries, the market is segmented into Germany, UK, France, Russia, Spain, Italy, and Rest of Europe.
By Application
By Deployment Mode
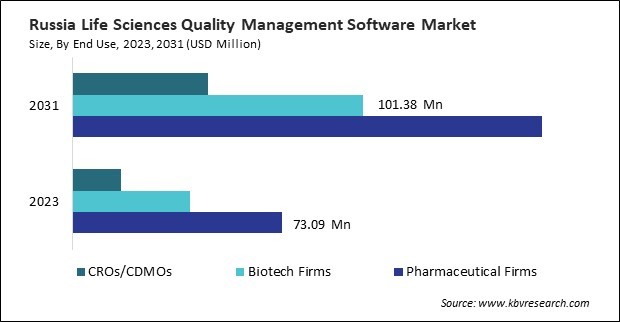
By End Use
By Country
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

