The Europe Regulatory Information Management System Market would witness market growth of 10.1% CAGR during the forecast period (2024-2031).
The Germany market dominated the Europe Regulatory Information Management System Market by Country in 2023, and would continue to be a dominant market till 2031; thereby, achieving a market value of $291.1 Million by 2031. The UK market is exhibiting a CAGR of 9.1% during (2024 - 2031). Additionally, The France market would experience a CAGR of 11% during (2024 - 2031).
RIMS provide tools for monitoring and analyzing regulatory developments and trends. They gather regulatory intelligence from various sources, such as regulatory agencies, industry publications, and news sources, to inform decision-making and strategy development. RIMS facilitate audit readiness by maintaining comprehensive records of regulatory activities, documentation, and compliance efforts. They provide documentation trails, audit logs, and reporting capabilities to demonstrate compliance with regulatory requirements during audits and inspections.
RIMS help companies identify, assess, and mitigate regulatory risks associated with non-compliance or adverse regulatory events. They enable risk assessment, risk monitoring, and risk mitigation strategies to minimize the impact of regulatory issues on business operations. RIMS supports quality management processes by integrating with quality management systems (QMS) and ensuring compliance with quality standards and regulations, such as Good Manufacturing Practice (GMP) or Good Clinical Practice (GCP).
France strongly emphasizes patient safety and quality in the healthcare system, requiring medical device companies to demonstrate the safety, efficacy, and performance of their products. Regulatory compliance processes, such as clinical evaluations, post-market surveillance, and quality management, require effective information management systems to track and monitor product safety and quality throughout the product lifecycle in France. Regulatory information management systems equipped with advanced features for adverse event monitoring, quality documentation, and compliance reporting enable medical device companies in France to maintain product safety and quality standards, demonstrate compliance with regulatory requirements, and respond effectively to regulatory inquiries and audits. Thus, all these factors will uplift the regional market’s expansion in the coming years.
Free Valuable Insights: The Global Regulatory Information Management System Market will Hit USD 4.7 Billion by 2031, at a CAGR of 10.5%
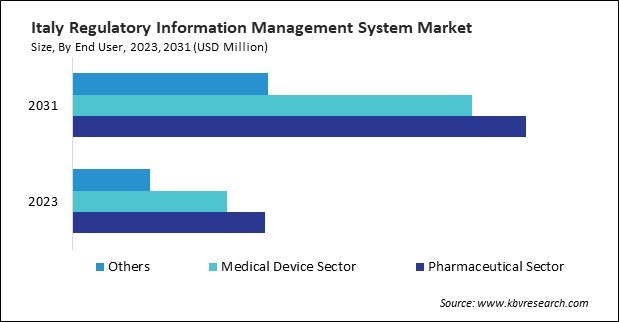
Based on End User, the market is segmented into Pharmaceutical Sector, Medical Device Sector, and Others. Based on countries, the market is segmented into Germany, UK, France, Russia, Spain, Italy, and Rest of Europe.
By End User
By Country
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

