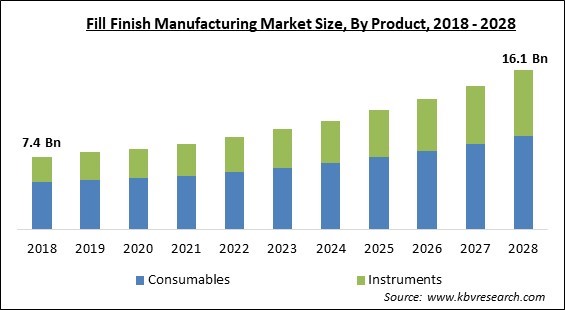
The Global Fill Finish Manufacturing Market size is expected to reach $16.1 billion by 2028, rising at a market growth of 9.5% CAGR during the forecast period.
The fill-finish step, which is probably the most important, brings the entire drug production process to a close. It comes after upstream bioprocessing, often known as the process of making the active ingredient through cell culture or fermentation. Additionally, it happens following downstream purification. Fill-Finish is a largely industrial activity that is still in its infancy but focuses on effective product composition and packaging.
Fill-finish involves intricate mechanical engineering filling, fluid, and solid pouring, and sealing systems—far more so than bioprocessing. A sterile medicine is delivered from filling needles to a sterile container, typically a vial or prefilled syringe, in an aseptic fill finish. The stopping (closing) of a container usually happens later, except in situations where medicine needs to be sterilely compressed.
It is a crucial step in the production of biopharmaceuticals. It is crucial to recognize how crucial these later-stage growth processes are. Innovation could be a silent killer, especially in a fill-finish process. But because fill-finish is so crucial to the biopharmaceutical production process, even the tiniest errors frequently result in product failure, which equals a total loss for a product that is already pricey.
Consumers depend on drugs like vaccines, nasal sprays, ear drops, and eye drops to be sterile, sanitary, and free from contamination almost every day. Parenteral products can have major negative effects, such as a fever or infection, if something non-sterile is added to the eye, or injected into muscle, skin, or vein. This is because parenteral products are given through injection or introduction to fragile mucus membranes.
COVID-19, and other recent pandemics and pandemics have brought to light the urgent need for vaccines and treatments to fight newly emerging and reemerging infectious illnesses. The manufacture of vaccines therefore urgently requires fill-finish services. Since CDMOs/CMOs often outsource fill-finish activities, these businesses are well-equipped to manage fill-finish of COVID-19 vaccinations as they begin to enter the market. Catalent is an example of a producer who can successfully grow up and meet demand of the market.
The uses of isolators and restricted-access barrier systems (RABS), which establish reliable sterile manufacturing environments and successfully isolate human operators from the fill-finish process, is a technological achievement in aseptic fill-finish operations. Barrier isolation systems, which are combined fill-finish machines capable of performing all steps of fill-finish processes, are being developed by many device manufacturers in the fill-finish manufacturing sector.
Manufacturers have been able to increase capabilities by utilizing technology aimed at reducing costs and amortizing capital investments over several products due to the emergence of biologic drugs regulatory paths. The need for locally produced biologics is being driven by rising living standards around the world as well as the desire of many governments to have enough vaccine and biologic manufacturing to service their populations.
Compared to non-sterile processes, aseptic fill-finish operations are more dangerous. For process execution, they need careful planning, qualified employees, and specialized facilities & equipment. Maintaining sterility is crucial because any contamination can result in significant financial losses and have a profound impact on the lives of patients. All of the components in aseptic fill-finishing operations must be sanitized before use. Sterility of the product is essential for assuring patient safety while using it.
Based on the Product, the Fill Finish Manufacturing Market is segmented into Consumables (Prefilled syringes, Vials, cartridges, and others), Instruments (Systems and machine types). The instruments segment witnessed a significant revenue share in the fill-finish manufacturing market in 2021. A variety of low-maintenance washers with a 1000-fold reduction in particles are available for production with modest to high throughput.
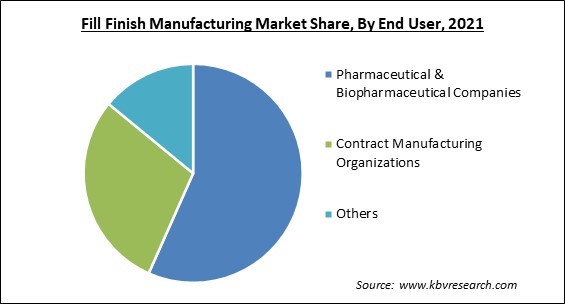
On the basis of End User, the Fill Finish Manufacturing Market is divided into Pharmaceutical & Biopharmaceutical Companies, Contract Manufacturing Organizations, and Other. The pharmaceutical & biopharmaceutical companies segment garnered the largest revenue share in the fill finish manufacturing market in 2021. The primary driver of market expansion is an increase in the production of pharmaceuticals and vaccines.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 8.6 Billion |
| Market size forecast in 2028 | USD 16.1 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 9.5% from 2022 to 2028 |
| Number of Pages | 304 |
| Number of Tables | 590 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Product, End User, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Region-wise, the Fill Finish Manufacturing Market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The Europe segment acquired the highest revenue share in the fill finish manufacturing market in 2021. By making biologics more affordable to use, factors like established reimbursement systems for medications have increased the demand for them among patients.
Free Valuable Insights: Global Fill Finish Manufacturing Market to reach USD 16.1 Billion by 2028
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Becton, Dickson, and Company, Gerresheimer AG, Syntegon Technology GmbH, West Pharmaceutical Services, Inc., OPTIMA packaging group GmbH, IMA Group, Marchesini Group S.p.A., Nipro Corporation, Schott AG (Carl-Zeiss-Stiftung), and SGD Pharma.
By End User
By Product
By Geography
The global Fill Finish Manufacturing Market size is expected to reach $16.1 billion by 2028.
Fill-Finish Manufacturing Techniques Are Advancing Technologically are driving the market in coming years, however, Maintaining Sterility In A Fill-Finish Manufacturing Operation Can Be Difficult restraints the growth of the market.
Becton, Dickson, and Company, Gerresheimer AG, Syntegon Technology GmbH, West Pharmaceutical Services, Inc., OPTIMA packaging group GmbH, IMA Group, Marchesini Group S.p.A., Nipro Corporation, Schott AG (Carl-Zeiss-Stiftung), and SGD Pharma.
The expected CAGR of the Fill Finish Manufacturing Market is 9.5% from 2022 to 2028.
The Consumables segment acquired maximum revenue share in the Global Fill Finish Manufacturing Market by Product in 2021 thereby, achieving a market value of $9.4 billion by 2028.
The Europe market dominated the Global Fill Finish Manufacturing Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $5.3 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

