Germany Mycoplasma Testing Market Size, Share & Trends Analysis Report By Product & Service (Kits & Reagents, Instruments and Services), By Technology, By Application, By End Germanyer, and Forecast, 2023 - 2030
Published Date : 20-May-2024 |
Pages: 75 |
Formats: PDF |
COVID-19 Impact on the Germany Mycoplasma Testing Market
The Germany Mycoplasma Testing Market size is expected to reach $116.84 Million by 2030, rising at a market growth of 9.7% CAGR during the forecast period.
The mycoplasma testing market in Germany has witnessed significant growth in recent years, driven by the increasing awareness about the importance of mycoplasma contamination control in various industries, including pharmaceuticals, biotechnology, and academic research. One of the key factors contributing to the growth of the mycoplasma testing market in Germany is the robust presence of the pharmaceutical and biotechnology sectors. Germany is home to several major pharmaceutical and biotech companies, and these industries heavily rely on cell cultures for drug development, vaccine production, and various research applications.
According to Germany Trade & Invest, Germany stands as a prominent global hub for pharmaceutical production, boasting a substantial increase in production volume, reaching EUR 34.6 billion in 2021 with a remarkable 6.9 % year-on-year growth. The subsequent year, 2022, witnessed a notable surge in pharmaceutical industry sales within Germany, marking 5.4 % and reaching EUR 56.5 billion at ex-manufacturer prices. This robust growth in Germany's pharmaceutical sector indicates the nation's pivotal role in the global pharmaceutical landscape.
In addition to the pharmaceutical and biotechnology sectors, academic research institutions in Germany play a pivotal role in driving the mycoplasma testing market. Research laboratories nationwide actively engage in diverse scientific disciplines, conducting experiments often involving cell cultures. The mycoplasma testing market in Germany is characterized by several key players offering a wide range of testing products and services. These include PCR-based assays, enzyme-linked immunosorbent assays (ELISAs), and other advanced molecular biology techniques.
Furthermore, regulatory agencies in Germany, such as the Federal Institute for Drugs and Medical Devices (BfArM) and the Paul-Ehrlich-Institut, emphasize the importance of mycoplasma testing in the production and quality control of pharmaceuticals and biological products. Compliance with these regulations has become a primary driver for the adoption of advanced mycoplasma testing technologies and services by industry players in Germany.
The impact of COVID-19 has been felt across various industries, including the mycoplasma testing market in Germany. The pandemic has led to disruptions in supply chains, laboratory operations, and research activities. While the demand for mycoplasma testing remains robust, the challenges posed by the pandemic have resulted in delays in testing processes and the adoption of new technologies. The focus on COVID-19 research and testing has also diverted resources, temporarily impacting the mycoplasma testing market. However, as the situation stabilizes and laboratories resume normal operations, the market is expected to regain momentum.
Market Trends
Rising adoption of advanced technologies in cell culture applications
In Germany, the mycoplasma testing market within the biotechnology field is experiencing a significant transformation due to the rising adoption of advanced technologies in cell culture applications. The German mycoplasma testing market is witnessing a surge in investments to develop innovative solutions for efficient and rapid detection of mycoplasma contaminants. The German mycoplasma testing market is witnessing a surge in investments to develop innovative solutions for efficient and rapid detection of mycoplasma contaminants.
German companies are focusing on research and development to stay at the forefront of technological advancements, catering to the biotechnology sector's evolving needs. Mycoplasma testing plays a crucial role in ensuring the quality and safety of biopharmaceutical products, as mycoplasma contamination can compromise the efficacy and safety of the final drug. In the context of the booming biosimilar industry in Germany, stringent mycoplasma testing becomes paramount to maintain the integrity of the development and manufacturing processes.
According to Germany Trade & Invest, in 2019, the pharmaceutical landscape in Germany saw significant activity, with 20 biosimilar products in development by various companies. The biosimilar industry exhibited an impressive CAGR of 69% during that period. This growth reflects biosimilars' increasing importance and acceptance in the pharmaceutical industry.
Collaborations between academia and industry players are also on the rise in Germany, fostering a collaborative environment that encourages the exchange of knowledge and expertise in mycoplasma testing. This collaborative approach accelerates developing and implementing advanced technologies in the biotechnology sector, strengthening Germany's position as a hub for biopharmaceutical innovation. Thus, Germany's mycoplasma testing market is undergoing a transformative phase driven by increased investments in innovative solutions and collaborative efforts between academia and industry players.
Increasing advancements in cell and gene therapies
Germany is at the forefront of advancements in cell and gene therapies, and this progress has significantly impacted the mycoplasma testing market within the country. As regenerative medicine continues to expand, ensuring the safety and efficacy of cell and gene therapies becomes paramount, driving the demand for robust mycoplasma testing solutions in Germany. One key driver of Germany's burgeoning mycoplasma testing market is the increasing investment in research and development of innovative therapies.
The country boasts a robust network of biotechnology and pharmaceutical companies actively developing cutting-edge cell and gene therapies. These therapies hold immense potential for treating various diseases, ranging from genetic disorders to cancer. The emphasis on quality control and regulatory compliance further fuels the need for advanced mycoplasma testing methodologies. German regulatory authorities maintain stringent standards to ensure the safety of therapeutic products. As a result, the mycoplasma testing market is witnessing a surge in the adoption of advanced testing technologies that offer high sensitivity and specificity.
Moreover, Germany's commitment to fostering an innovation-friendly environment and its well-established infrastructure contribute to the rapid growth of the mycoplasma testing market. The availability of state-of-the-art laboratories and skilled professionals further supports implementing sophisticated testing protocols. Therefore, Germany's leadership in cell and gene therapies, robust R&D investments, stringent regulatory standards, and an innovation-friendly environment propel the mycoplasma testing market's rapid growth.
Competition Analysis
The mycoplasma testing market in Germany is characterized by several key companies that play a crucial role in ensuring the quality and safety of biological products, especially in pharmaceuticals, biotechnology, and research. One prominent player in the German mycoplasma testing market is Merck KGaA. As a global science and technology company, Merck provides a comprehensive range of products and services, including mycoplasma testing solutions. Their innovative technologies aid researchers and manufacturers in detecting and eliminating mycoplasma contamination, ensuring the integrity of cell cultures and biological products.
Sartorius AG is a significant player providing mycoplasma testing solutions tailored to the German mycoplasma testing market. Their expertise in bioprocessing and laboratory technologies extends to mycoplasma detection, ensuring the safety of biopharmaceutical production processes. Sartorius's commitment to quality control aligns with the stringent regulatory standards in Germany, making it a trusted partner for mycoplasma testing solutions.
Another key participant in the German mycoplasma testing market is Roche Diagnostics. Renowned for its diagnostic solutions, Roche Diagnostics offers advanced tools for mycoplasma detection. These solutions are pivotal for maintaining the quality of cell lines and ensuring the reliability of experimental results in various life science applications. The company's commitment to precision and efficiency aligns with the stringent requirements of the German mycoplasma testing landscape.
Qiagen, a global provider of sample and assay technologies, is actively involved in the German mycoplasma testing market. The company's products address the complexities of mycoplasma detection in cell cultures, contributing to research outcomes' reliability and biopharmaceutical products' safety. Qiagen's commitment to innovation and scientific rigor resonates well with Germany's research and biotechnology communities. These companies contribute to advancing mycoplasma detection technologies, aligning with the German biotechnology and pharmaceutical sectors' stringent regulatory standards and quality control requirements.
List of Key Companies Profiled
- Charles River Laboratories International, Inc.
- Thermo Fisher Scientific, Inc.
- Eurofins Scientific SE
- Lonza Group Ltd.
- Bio-Rad laboratories, Inc.
- InvivoGen SAS
- Asahi Kasei Corporation
- F.Hoffmann-La Roche Ltd.
- Norgen Biotek Corp.
- PromoCell GmbH
Germany Mycoplasma Testing Market Report Segmentation
By Product & Service
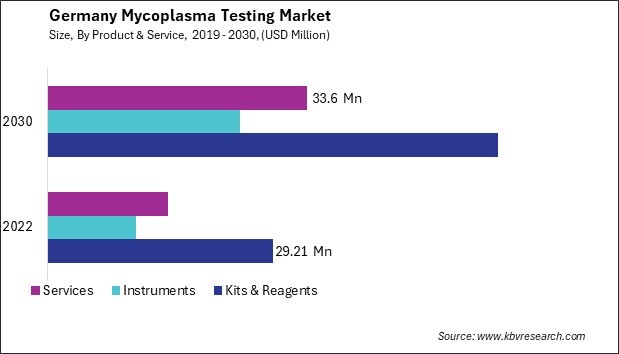
- Kits & Reagents
- Instruments
- Services
By Technology
- PCR
- ELISA
- Microbial Culture Techniques
- Enzymatic Methods
By Application
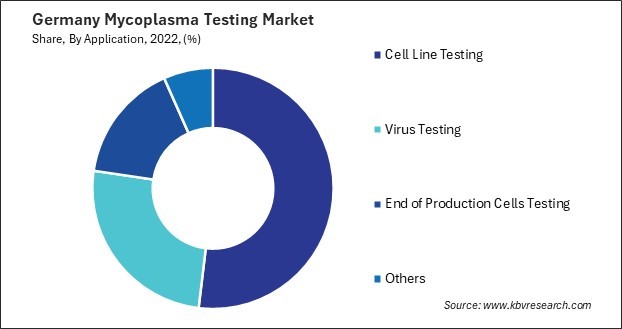
- Cell Line Testing
- Virus Testing
- End of Production Cells Testing
- Others
By End User
- Pharmaceutical & Biotechnology Companies
- Contract Research Organizations
- Academic Research Institutes
- Cell Banks
- Others
1.1 Market Definition
1.2 Objectives
1.3 Market Scope
1.4 Segmentation
1.4.1 Germany Mycoplasma Testing Market, by Product & Service
1.4.2 Germany Mycoplasma Testing Market, by Technology
1.4.3 Germany Mycoplasma Testing Market, by Application
1.4.4 Germany Mycoplasma Testing Market, by End User
1.5 Methodology for the research
Chapter 2. Market Overview
2.1 Introduction
2.1.1 Overview
2.1.1.1 Market Composition and Scenario
2.2 Key Factors Impacting the Market
2.2.1 Market Drivers
2.2.2 Market Restraints
2.2.3 Market Trends
2.3 Porter Five Forces Analysis
Chapter 3. Germany Mycoplasma Testing Market
3.1 Germany Mycoplasma Testing Market by Product & Service
3.2 Germany Mycoplasma Testing Market by Technology
3.3 Germany Mycoplasma Testing Market by Application
3.4 Germany Mycoplasma Testing Market by End User
Chapter 4. Company Profiles – Global Leaders
4.1 Charles River Laboratories International, Inc.
4.1.1 Company Overview
4.1.2 Financial Analysis
4.1.3 Segmental and Regional Analysis
4.2 Thermo Fisher Scientific, Inc.
4.2.1 Company Overview
4.2.2 Financial Analysis
4.2.3 Segmental and Regional Analysis
4.2.4 Research & Development Expenses
4.2.5 SWOT Analysis
4.3 Eurofins Scientific SE
4.3.1 Company Overview
4.3.2 Financial Analysis
4.3.3 Regional Analysis
4.3.4 Recent strategies and developments:
4.3.4.1 Product Launches and Product Expansions:
4.3.4.2 Acquisition and Mergers:
4.3.5 SWOT Analysis
4.4 Lonza Group Ltd.
4.4.1 Company Overview
4.4.2 Financial Analysis
4.4.3 Segmental and Regional Analysis
4.4.4 Research & Development Expenses
4.4.5 SWOT Analysis
4.5 Bio-Rad laboratories, Inc.
4.5.1 Company Overview
4.5.2 Financial Analysis
4.5.3 Segmental and Regional Analysis
4.5.4 Research & Development Expenses
4.5.5 SWOT Analysis
4.6 InvivoGen SAS
4.6.1 Company Overview
4.6.2 SWOT Analysis
4.7 Asahi Kasei Corporation
4.7.1 Company Overview
4.7.2 Financial Analysis
4.7.3 Segmental and Regional Analysis
4.7.4 Research & Development Expenses
4.7.5 SWOT Analysis
4.8 F. Hoffmann-La Roche Ltd.
4.8.1 Company Overview
4.8.2 Financial Analysis
4.8.3 Segmental and Regional Analysis
4.8.4 Research & Development Expense
4.8.5 SWOT Analysis
4.9 Norgen Biotek Corp.
4.9.1 Company Overview
4.10. PromoCell GmbH
4.10.1 Company Overview
4.10.2 SWOT Analysis
TABLE 2 Germany Mycoplasma Testing Market, 2023 - 2030, USD Million
TABLE 3 Germany Mycoplasma Testing Market by Product & Service, 2019 - 2022, USD Million
TABLE 4 Germany Mycoplasma Testing Market by Product & Service, 2023 - 2030, USD Million
TABLE 5 Germany Mycoplasma Testing Market by Technology, 2019 - 2022, USD Million
TABLE 6 Germany Mycoplasma Testing Market by Technology, 2023 - 2030, USD Million
TABLE 7 Germany Mycoplasma Testing Market by Application, 2019 - 2022, USD Million
TABLE 8 Germany Mycoplasma Testing Market by Application, 2023 - 2030, USD Million
TABLE 9 Germany Mycoplasma Testing Market by End User, 2019 - 2022, USD Million
TABLE 10 Germany Mycoplasma Testing Market by End User, 2023 - 2030, USD Million
TABLE 11 Key Information – Charles River Laboratories International, Inc.
TABLE 12 Key Information – Thermo Fisher Scientific, Inc.
TABLE 13 Key Information – Eurofins Scientific SE
TABLE 14 Key Information –Lonza Group Ltd.
TABLE 15 Key Information – Bio-Rad Laboratories, Inc.
TABLE 16 Key Information – InvivoGen SAS
TABLE 17 Key Information – Asahi Kasei Corporation
TABLE 18 Key Information – F. Hoffmann-La Roche Ltd.
TABLE 19 Key Information – Norgen Biotek Corp.
TABLE 20 Key Information – PromoCell GmbH
List of Figures
FIG 1 Methodology for the research
FIG 2 Germany Mycoplasma Testing Market, 2019 - 2030, USD Million
FIG 3 Key Factors Impacting Mycoplasma Testing Market
FIG 4 Porter’s Five Forces Analysis – Mycoplasma Testing Market
FIG 5 Germany Mycoplasma Testing Market share by Product & Service, 2022
FIG 6 Germany Mycoplasma Testing Market share by Product & Service, 2030
FIG 7 Germany Mycoplasma Testing Market by Product & Service, 2019 - 2030, USD Million
FIG 8 Germany Mycoplasma Testing Market share by Technology, 2022
FIG 9 Germany Mycoplasma Testing Market share by Technology, 2030
FIG 10 Germany Mycoplasma Testing Market by Technology, 2019 - 2030, USD Million
FIG 11 Germany Mycoplasma Testing Market share by Application, 2022
FIG 12 Germany Mycoplasma Testing Market share by Application, 2030
FIG 13 Germany Mycoplasma Testing Market by Application, 2019 - 2030, USD Million
FIG 14 Germany Mycoplasma Testing Market share by End User, 2022
FIG 15 Germany Mycoplasma Testing Market share by End User, 2030
FIG 16 Germany Mycoplasma Testing Market by End User, 2019 - 2030, USD Million
FIG 17 SWOT Analysis: Thermo Fisher Scientific, Inc.
FIG 18 SWOT Analysis: Eurofins Scientific SE
FIG 19 SWOT Analysis: Lonza Group Ltd.
FIG 20 SWOT Analysis: Bio-Rad Laboratories, Inc.
FIG 21 SWOT Analysis: InvivoGen SAS
FIG 22 SWOT Analysis: Asahi Kasei Corporation
FIG 23 SWOT Analysis: F. Hoffmann-La Roche Ltd.
FIG 24 SWOT Analysis: PromoCell GmbH