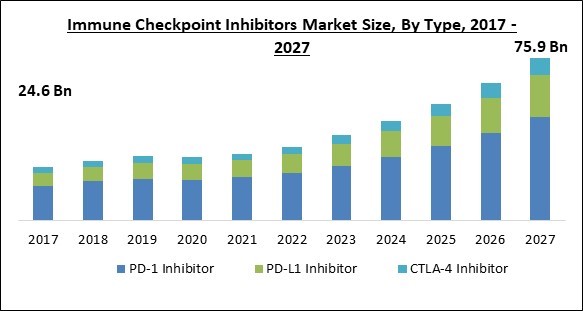
The Global Immune Checkpoint Inhibitors Market size is expected to reach $75.9 billion by 2027, rising at a market growth of 16.1% CAGR during the forecast period.
A checkpoint inhibitor is a kind of immunotherapy medicine that works by blocking proteins found on tumor cells that interfere with immune system activity. CTLA-4 (cytotoxic T lymphocyte-associated protein 4), PD-1 (programmed cell death protein 1), and PD-L1 are all immune checkpoint proteins that are blocked by immune checkpoint inhibitors (programmed death-ligand 1). Immune checkpoint inhibitors work by assisting the immune system in recognizing and attacking cancer cells by targeting checkpoint proteins.
In addition, immune checkpoint inhibitors work by assisting the immune system in recognizing and attacking cancer cells by targeting checkpoint proteins. Due to an increase in demand for optimum therapies for cancer treatment, advantageous payment policies provided by manufacturers and insurance providers in some countries, and an increase in cancer occurrence around the world, the demand for immune checkpoint inhibitors is increasing.
COVID-19 Pandemic was first recorded in 2019 in China, after the first appearance, the novel coronavirus rapidly spread across the world and caused severe harm to people as well as businesses. Patients experience a variety of symptoms as a result of this infection, ranging from minor to severe. Dry cough, fever, and exhaustion are some of the most prevalent symptoms. Breathing difficulties or difficulty in breathing, chest pain or pressure, and impairment of speech or movement are all dangerous signs. Moreover, the virus has a significant fatality potential in the geriatric population.
Additionally, COVID-19 has had a negative overall impact on the immune checkpoint inhibitors market, owing to a decrease in the number of cancer patients visiting hospitals and clinics for immune checkpoint inhibitor therapy (ICI), which has resulted in a decrease in demand for immune checkpoint inhibitor products.
Lung cancer has a terrible prognosis and is the biggest cause of cancer-related death worldwide. Progression-free survival (PFS) and survival rate are both restricted despite vigorous treatment. Immune checkpoint inhibitors (ICIs) targeting PD1, PDL1, and CTLA-4 have recently been available for a variety of malignancies. ICIs have transformed the treatment of cancer, including lung cancer, and have made a huge breakthrough. However, there are numerous debates about whether patients are best treated with ICIs in terms of monotherapy, combination therapy, and predictive biomarkers.
Immune checkpoint inhibitors have been found to be effective in the treatment of a variety of cancers, including breast, bladder, stomach, colon, liver, cervical, stomach, lung, skin, stomach, and rectal cancer.
The increasing prevalence of the geriatric population across the world is one of the major concerns of healthcare infrastructures globally. As the age of a person increases, it makes their immune systems more fragile to various kinds of cancers, especially prostate cancer and lung cancer. The geriatric population is majorly more vulnerable to chronic diseases. As per the data shared by the United Nations Organization, in 2019, 703 million people of age 65 or more than that were recorded across the world. Moreover, this number is estimated to reach 1.5 billion by 2050 by the UNO.
Cancer is a disease that are of various types, which can begin in practically any organ or tissue of the body and spread to other organs when abnormal cells proliferate uncontrollably, and invade surrounding areas of the body, and/or move to other organs. It is a very fatal disorder that can end up in life losses. One of the major concerns among healthcare practitioners across the world is that a significant population is not fully aware of the prevalence of various types of cancer, which further delays the diagnosis and hence, increases the death rate.
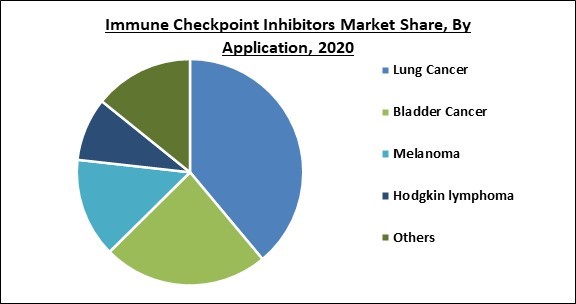
Based on Application, the market is segmented into Lung Cancer, Bladder Cancer, Melanoma, Hodgkin lymphoma, and others. In 2020, the bladder cancer segment registered a significant revenue share in the immune checkpoint inhibitors market. The growth of this segment is increasing as a result of the high prevalence of bladder cancer and advances in diagnostic procedures. In addition, both pembrolizumab and atezolizumab are immune checkpoint inhibitors that have been approved by the FDA to treat patients with metastatic bladder cancer who have progressed after traditional treatments such as chemotherapy medication combinations.
Based on Type, the market is segmented into PD-1 Inhibitor, PD-L1 Inhibitor, and CTLA-4 Inhibitor. In 2020, The PD-1 inhibitors segment dominated the market by acquiring the largest revenue share of the immune checkpoint inhibitors market. The PD-1 inhibitors are being adopted increasingly due to their efficiency and increase in the prescriptions of nivolumab and pembrolizumab. In addition, pembrolizumab (KEYTRUDA), the first PD-1 inhibitor, has seen increased adoption due to its proven efficacy in treating multiple FDA-approved indications such as melanoma, non-small cell lung cancer (NSCLC), head and neck squamous cell cancer (HNSCC), and others, all of which are highly prevalent globally, and thus, driving the segment growth.
| Report Attribute | Details |
|---|---|
| Market size value in 2020 | USD 29.3 Billion |
| Market size forecast in 2027 | USD 75.9 Billion |
| Base Year | 2020 |
| Historical Period | 2017 to 2019 |
| Forecast Period | 2021 to 2027 |
| Revenue Growth Rate | CAGR of 16.1% from 2021 to 2027 |
| Number of Pages | 185 |
| Number of Tables | 283 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Type, Application, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. Europe acquired a substantial revenue share of the immune checkpoint inhibitors market in 2020. This segment is rapidly flourishing due to the complete availability of immune checkpoint inhibitors around the region. All of the seven immune checkpoint inhibitors viz. pembrolizumab, nivolumab, durvalumab, atezolizumab, avelumab, ipilimumab, and cemiplimab are available in this region.
Free Valuable Insights: Global Immune Checkpoint Inhibitors Market size to reach USD 75.9 Billion by 2027
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include AstraZeneca PLC, Beigene Ltd., Shanghai Junshi Biosciences Co., Ltd., Bristol Myers Squibb Company, GlaxoSmithKline PLC, Eli Lilly and Company, Merck Group (Merck Sharp & Dohme Corp.), Sanofi S.A., Merck & Co., Inc., and F. Hoffmann-La Roche Ltd.
By Application
By Type
By Geography
The global immune checkpoint inhibitors market size is expected to reach $75.9 billion by 2027.
Increasing prevalence of cancer across the world are driving the market in coming years, however, Lack of awareness among people limited the growth of the market.
AstraZeneca PLC, Beigene Ltd., Shanghai Junshi Biosciences Co., Ltd., Bristol Myers Squibb Company, GlaxoSmithKline PLC, Eli Lilly and Company, Merck Group (Merck Sharp & Dohme Corp.), Sanofi S.A., Merck & Co., Inc., and F. Hoffmann-La Roche Ltd.
The Lung Cancer segment is leading the Global Immune Checkpoint Inhibitors Market by Application 2020, and would continue to be a dominant market till 2027.
The PD-L1 Inhibitor segment has high growth rate of 16.6% during (2021 - 2027).
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

