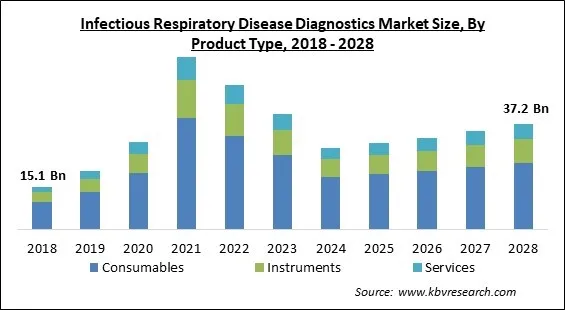
The Global Infectious Respiratory Disease Diagnostics Market size is expected to reach $37.2 billion by 2028, rising at a market growth of -5.2% CAGR during the forecast period.
The condition that affects the lungs and respiratory system is referred to as a respiratory disease. It is brought on by an infection, tobacco usage, or air pollution. Among the most common respiratory illnesses are chronic obstructive pulmonary disease (COPD), lung cancer, and asthma. The two main reasons propelling the market's expansion are the rising number of elderly people with respiratory problems and technical advancements in respiratory diagnostic tests. The overall market growth for respiratory illness testing/diagnostics is also expected to be impacted by the rising prevalence of respiratory disease associated with rising rates of cigarette use.
Infectious respiratory disorders like tuberculosis and pneumonia are becoming more common, diagnostic technology is improving, and significant companies, the government, and charitable groups are investing more in R&D for diagnostic testing. Additionally, increased number of product launches along with increased demand for tests and consumables during the pandemic. These are the main reasons that will drive the market.
Rapid technology improvements are anticipated to have a significant impact on this market by enabling accurate findings, portability, and cost-effectiveness. Due to the comparable symptoms of RSV, COVID-19, and influenza, major players are investing in the R&D and commercialization of multiplex tests for their use in the simultaneous diagnosis of these illnesses. Multiplex testing makes it possible to target numerous genes at once, cutting down on the time and expense associated with performing traditional PCR. Self-testing and point-of-care solutions are becoming more widely used, which might help the market develop even more.
The need for infectious disease diagnostics products has increased due to the rising burden of infectious illnesses and hospital-acquired infections worldwide. Many people dealt with respiratory issues due to the COVID-19 effect. Although many committed healthcare workers are working tirelessly on the front lines to combat this disaster, it's equally crucial to prevent the spread of disease by getting a diagnosis as soon as possible. This is projected to have an impact on the demand for quick diagnostics testing and grow the respiratory infectious diagnostics market throughout the projection period.
The proportion of elderly people in the population is rising in almost every nation on earth. Around 703 million people around the world were 65 or older in 2019. By 2050, there would be 1.5 billion older people in the world, a more than double increase. One of the main drivers of the growth of the market for respiratory disease testing and diagnostics is the increase in the world's geriatric population. A large number of old age people are suffering from diseases such as TB. Also, the pulmonary disease is now one of the most common causes of death worldwide.
Rising investment in research & development, particularly in developed and developing economies, will further open up lucrative market expansion opportunities for medical instruments and devices. One another significant factor promoting the growth of the market is the increased focus on improving the state of healthcare facilities and the infrastructure for healthcare as a whole. The public-private partnerships and strategic collaborations for the purpose of funding and implementing new and improved technology has been increased over the past few years.
Due to the concentration of patients with lung diseases, the potential for coughing, and the formation of droplets around pulmonary function test procedures, pulmonary function testing could be a route for the transmission of infection. These systems include laboratory-grade tubing, rebreathing valves, and mouthpieces. The most frequent source of cross-infection during testing is mouthpieces. The risk of developing tuberculosis increases due to factors such as exposed surface areas, upholstery quality, air conditioning, general clutter, ideal temperature, and humidity levels.
Based on product type, the infectious respiratory disease diagnostics market is segmented into instruments, consumables and services. The instrument segment acquired a substantial revenue share in the infectious respiratory disease diagnostics market in 2021. This is because of the development of products that are cutting-edge and help doctors diagnose COVID-19 patients. Furthermore, the demand for respiratory disease diagnostics instruments is rising as the patients are now more aware of consequences of such diseases and thus are more concerned for fitness.
Under instruments type, the infectious respiratory disease market is divided into imaging tests, respiratory measurement devices and other instruments. The imaging test segment garnered maximum revenue share in the infectious respiratory disease diagnostics market in 2021. A high-resolution CT scan could provide more information on lung problems. Images in three dimensions are possible with helical CT. Typically, a big breath is taken before a CT scan (inhales).
By application, the infectious respiratory disease diagnostics market is divided into SARS/COVID-19, influenza, RSV (Respiratory Syncytial Virus), tuberculosis, streptococcus testing and other respiratory disease testing. In 2021, the SARS/COVID-19 segment witnessed the largest revenue share in the infectious respiratory disease diagnostics market. The dominance is the result of the high incidence of COVID-19, more product approvals, and greater R&D. Nevertheless, during the predicted period, rising immunization rates are anticipated to lessen the disease's severity and declining testing rates.
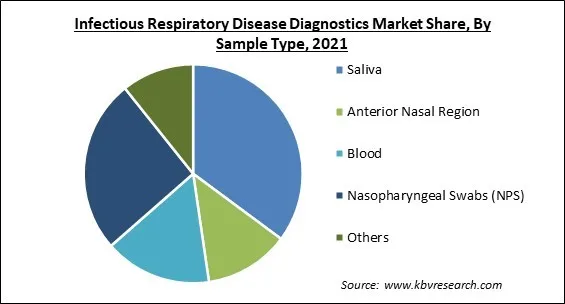
On the basis of sample type, the infectious respiratory disease diagnostics market is fragmented into saliva, nasopharyngeal swabs (NPS), anterior nasal region, blood and others. In 2021, the nasopharyngeal swabs segment recorded a substantial revenue share in the infectious respiratory disease diagnostics market. This growth is the result of the increase in demand for NPS accompanied by a wide range of applications for conducting various tests, such as rapid antigen detection tests, direct fluorescent antibodies, and Polymerase Chain Reaction (PCR). Many businesses have dramatically increased their swab manufacturing capacities in response to the COVID-19 pandemic.
Based on technology, the infectious respiratory disease diagnostics market is classified into immunoassay, molecular diagnostics, micro-biology and other technologies. In 2021, the molecular diagnostics segment accounted the maximum revenue share in the infectious respiratory disease diagnostics market. The rising demand for RT-PCR testing for the diagnosis of COVID-19, RSV, influenza, and other diseases might be blamed for the high share. The most well-known and dependable gold standard technology for amplifying DNA to undertake molecular diagnostics is a polymerase chain reaction.
On the basis of end-use, the infectious respiratory disease diagnostics market is segmented into hospitals, diagnostic laboratories, physician offices, and other end users. The physician offices segment generated a significant revenue share in the infectious respiratory disease diagnostics market in 2021. This is due to growing patient preference and manufacturers' increased attention on the development of point-of-care diagnostics, the physician offices end-use sector is anticipated to see a high growth rate over the course of the forecast period. It is anticipated that the advent of innovative assays that deliver speedy PoC findings would accelerate segment expansion.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 61 Billion |
| Market size forecast in 2028 | USD 37.2 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of -5.2% from 2022 to 2028 |
| Number of Pages | 417 |
| Number of Tables | 685 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Product Type, Application, Sample Type, Technology, End-use, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Region wise, the infectious respiratory disease diagnostics market is analysed across the North America, Europe, Asia Pacific and LAMEA. In 2021, the Asia Pacific region led the infectious respiratory disease diagnostics market by generating the highest revenue share. The market in this region is being propelled due to the rising number of the ageing population. Furthermore, the government of nations in the region such as India and China is taking various initiatives in order to enhance the healthcare facilities in the region. As a result of this, the infectious respiratory disease diagnostics market would grow in the region.
Free Valuable Insights: Global Infectious Respiratory Disease Diagnostics Market size to reach USD 37.2 Billion by 2028
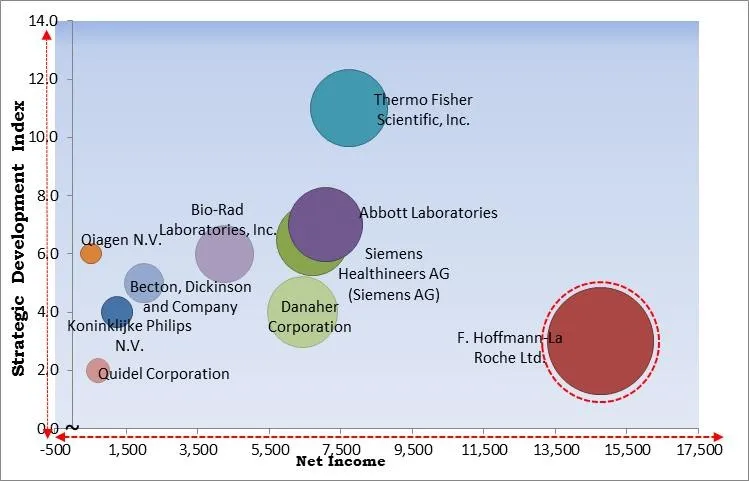
The major strategies followed by the market participants are Product Launches. Based on the Analysis presented in the Cardinal matrix; F. Hoffmann-La Roche Ltd. is the forerunners in the Infectious Respiratory Disease Diagnostics Market. Companies such as Thermo Fisher Scientific, Inc., Abbott Laboratories and Siemens Healthineers AG are some of the key innovators in Infectious Respiratory Disease Diagnostics Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Abbott Laboratories, Becton, Dickinson and Company, Siemens Healthineers AG (Siemens AG), Thermo Fisher Scientific, Inc., Koninklijke Philips N.V., F. Hoffmann-La Roche Ltd., Qiagen N.V., Bio-Rad Laboratories, Inc., Danaher Corporation, Quidel Corporation
By Product Type
By Application
By Sample Type
By Technology
By End Use
By Geography
The global Infectious Respiratory Disease Diagnostics Market size is expected to reach $37.2 billion by 2028.
Increase in Aged Population are driving the market in coming years, however, Cross-Contamination Dangers and a Lack of Qualified Medical Personnel restraints the growth of the market.
Abbott Laboratories, Becton, Dickinson and Company, Siemens Healthineers AG (Siemens AG), Thermo Fisher Scientific, Inc., Koninklijke Philips N.V., F. Hoffmann-La Roche Ltd., Qiagen N.V., Bio-Rad Laboratories, Inc., Danaher Corporation, Quidel Corporation
The Saliva market acquired maximum revenue share in Global Infectious Respiratory Disease Diagnostics Market by Sample Type in 2021; thereby, achieving a market value of $12.4 billion by 2028.
The Consumables market is leading the Global Infectious Respiratory Disease Diagnostics Market by Product Type in 2021; thereby, achieving a market value of $23.6 billion by 2028.
The Asia Pacific market dominated the Global Infectious Respiratory Disease Diagnostics Market by Region in 2021; thereby, achieving a market value of $13.0 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

