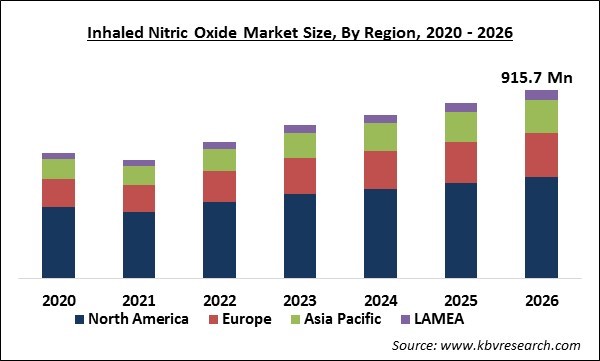
The Global Inhaled Nitric Oxide Market size is expected to reach $915.7 Million by 2026, rising at a market growth of 7% CAGR during the forecast period. Nitric oxide is a molecule of gas that causes a sense of relaxation in smooth muscle cells in the vasculature. It is because Nitric Oxide reacts with oxyhemoglobin. The gas is quickly collected by oxyhemoglobin in red blood cells and the vasodilating effects caused by inhaled nitric oxide are restricted to oxygenated areas of the lungs. Thus, this distinct ability of Nitric oxide to cause pulmonary vasodilatation in the oxygenated regions of the lung helps in enhancing oxygenation of the blood and reduces intrapulmonary right to left shunting. Hence, the inhaled nitric oxide is utilized to cure various cardiopulmonary conditions, like pulmonary hypertension in adults and children. Although, the usage of inhaled nitric oxide is restricted due to logistical and financial reasons.
The rising prevalence of diseases in infants like a neonatal hypoxic respiratory failure (HRF) and persistent pulmonary hypertension is among the key driving factors of the market. Moreover, the growing number of patients with chronic obstructive pulmonary disease (COPD), and acute respiratory distress syndrome (ARDS) also propel the market growth. Apart from this, the huge cost involved in the treatment & the stringent government policies on different applications of the gas are restricting the growth of Inhaled Nitric Oxide market. However, the constant R&D activities for discovering various new applications in the healthcare sector are estimated to offer profitable opportunities for expanding the global inhaled nitric oxide market during the forecast period.
The declaration of WHO about the Covid-19 outbreak as a public health emergency has affected every country around the globe. During the pandemic, nitric oxide got under several experiments to test whether it can help in treating Covid-19 affected people, due to its antiviral properties and enhanced oxygenation. Inhaled Nitric Oxide is useful in safeguarding the frontline healthcare workers from this pandemic.
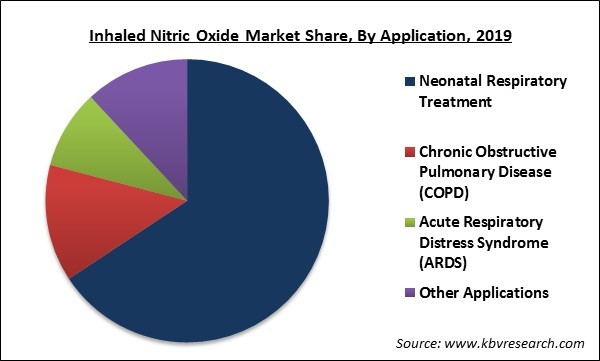
Based on Application, the market is segmented into Neonatal Respiratory Treatment, Chronic Obstructive Pulmonary Disease (COPD), Acute Respiratory Distress Syndrome (ARDS) and Other Applications. Neonatal respiratory treatment segment procured the substantial revenue share and is estimated to exhibit high growth rate during the forecast period. This is due a surge in the occurrences of diseases in the newborn like persistent pulmonary hypertension, and neonatal hypoxic respiratory failure (HRF). The chronic obstructive pulmonary disease segment would display promising growth rate during the forecast period.
| Report Attribute | Details |
|---|---|
| Market size value in 2019 | USD 581.5 Million |
| Market size forecast in 2026 | USD 915.7 Million |
| Base Year | 2019 |
| Historical Period | 2016 to 2018 |
| Forecast Period | 2020 to 2026 |
| Revenue Growth Rate | CAGR of 7% from 2020 to 2026 |
| Number of Pages | 123 |
| Number of Tables | 180 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Application, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Australia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Free Valuable Insights: Global Inhaled Nitric Oxide Market to reach a market size of $915.7 million by 2026
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. North America emerged as the leading region in the global inhaled nitric oxide market in 2019. The high growth of the regional market is due to the large consumer base & rising funding in R&D by the leading players. Major determinants such as surge in rates of chronic diseases in the newborn like persistent pulmonary hypertension, and neonatal hypoxic respiratory failure (HRF) are expected to support the market growth.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Getinge AB, Merck Group, Halma PLC, Linde PLC (Praxair, Inc.), Air Liquide S.A., Beyond Air, Inc., Mallinckrodt PLC (International Minerals and Chemical Corporation), VERO Biotech LLC, Nu-Med Plus, Inc. and Novoteris, LLC.
By Application
By Geography
The global inhaled nitric oxide market size is expected to reach $915.7 million by 2026.
There are several reason that cause high demand of this market one of them is increasing cases of congenital heart disease.
The Neonatal Respiratory Treatment market dominated the Global Inhaled Nitric Oxide Market by Application in 2019.
The North America market dominated the Global inhaled nitric oxide Market by Region in 2019.
Getinge AB, Merck Group, Halma PLC, Linde PLC (Praxair, Inc.), Air Liquide S.A., Beyond Air, Inc., Mallinckrodt PLC (International Minerals and Chemical Corporation), VERO Biotech LLC, Nu-Med Plus, Inc. and Novoteris, LLC.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

