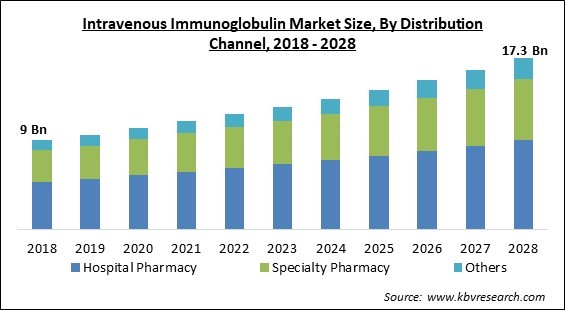
The Global Intravenous Immunoglobulin Market size is expected to reach $17.3 billion by 2028, rising at a market growth of 6.9% CAGR during the forecast period.
Immunoglobulins are glycoprotein molecules that are produced by plasma or white blood cells. It plays a crucial role in the immune system's defense by identifying and attaching to certain antigens such as bacteria and viruses, assisting in the elimination. Intravenous immunoglobulins are immunoglobulins that are given via intravenous injection. This is because most patients receiving immunoglobulins are in hospitals or clinical settings, and intravenous immunoglobulins are the most prevalent mode of administration. This coupled with the demonstrated efficacy of the immunoglobulins as an efficient therapy choice for a number of immunological illnesses and the inadequate amount of training for home-care settings is also positively influencing the Intravenous Immunoglobulin market growth.
Several people may use IVIg instead of other medications to treat their immune system problems such as immunosuppressants, corticosteroids, or biological therapies. IVIg may be used in conjunction with immunosuppressants or other medications in some instances. The immune system of the body regularly produces enough antibodies to fight infection-causing bacteria. The body can't create enough of them if a patient has an immunological deficit. As a result, the patient is more susceptible to infections that can make a person very unwell. IVIg provides antibodies that the body doesn't produce on its own, allowing the patient to fight infections.
The medication may help the body raise low red blood-cell levels in autoimmune illnesses like lupus. If a person doesn't get enough of them, the person suffers from anaemia and weary. IVIg prevents lupus patients' white blood cells from damaging the red blood cells. The medication may stop the patient's immune system from destroying muscle cells if such a person has myositis.
The COVID-19 pandemic has presented both a threat and a lucrative opportunity for the IVIG business. The pandemic has also brought together a number of significant players and governments to support the development of novel COVID-19 hyper-immunoglobulins. Plasma-based medicines are being researched for their effectiveness in reducing problems due to severe acute viral respiratory infections, comprising COVID-19. This medication is derived from recovered patients' plasma. The market is likely to be driven by the pandemic, which is presently in its last phases. Many people with symptomatic secondary immunodeficiency (SID), auto-inflammatory disorders, primary immunodeficiency (PID), and C1 inhibitor deficiency were infected with the SARS-CoV-2 virus in the United Kingdom, some were hospitalized to hospitals, and few patients died.
The medical system is witnessing the increasing immunoglobulin use, as well as a growth in the frequency of new launches and quick approval from government regulatory authorities around the world. For instance, as per the National Institutes of Health, a clinical trial to assess the safety, tolerability, and efficacy of a combination treatment regimen for coronavirus disease that includes the antiviral Remdesivir plus a highly concentrated solution of antibodies that neutralize SARS-CoV-2.The phase 3 trial, termed Inpatient Treatment with Anti-Coronavirus Immunoglobulin, or ITAC, was sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID), which is part of the National Institutes of Health.
Primary humoral immunodeficiency, chronic inflammatory demyelinating polyneuropathy (CIDP), idiopathic thrombocytopenic purpura (ITP), Guillain-Barre syndrome, multifocal motor neuropathy (MMN), myasthenia gravis, Kawasaki disease, hypogammaglobulinemia, chronic lymphocytic leukemia, and others are among the indications for intravenous immunoglobulins. The medical system witnessed a rise in the prevalence of primary immunodeficiency disorders (PID) over the world. In patients with lymphoproliferative diseases, it is the most frequent chronic immunological deficiency (LPDs).
The risk of contracting a blood-borne infection from blood donors who provide IVIG infusions is very minimal. This is due to the purifying procedure used to purify given blood plasma, which aids in the destruction of infectious organisms such as bacteria and viruses. Additionally, once the purification process is completed, IVIG donations are maintained and packed in sterilized packaging. This aids in the prevention of infectious disease transmission even more. Aseptic meningitis, a kind of non-infection-related brain inflammation, is a significant side effect of IVIG therapy.
Based on Distribution Channel, the market is segmented into Hospital Pharmacy, Specialty Pharmacy, and Others. The Speciality Pharmacy segment procured a substantial revenue share in the intravenous immunoglobulin market in 2021. This is because specialist pharmacies provide convenient treatment at home. Specialty medications are a vital and growing element of the pharmacy industry. As a result, the number of licensed specialty pharmaceuticals is fast expanding, propelling the specialty pharmacy industry forward over the forecast period.
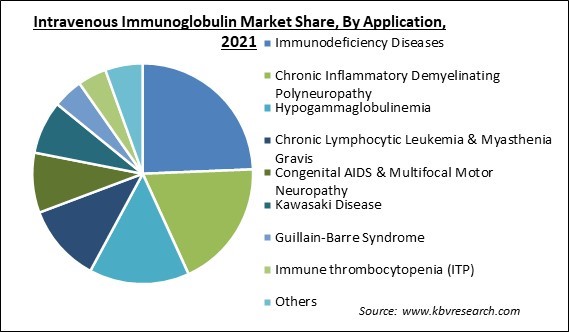
Based on Application, the market is segmented into Immunodeficiency Diseases, Chronic Inflammatory Demyelinating Polyneuropathy, Hypogammaglobulinemia, Chronic Lymphocytic Leukemia & Myasthenia Gravis, Congenital AIDS & Multifocal Motor Neuropathy, Kawasaki Disease, Guillain-Barre Syndrome, Immune thrombocytopenia (ITP), and Others. The chronic inflammatory demyelinating polyneuropathy segment garnered a significant revenue share in the intravenous immunoglobulin market in 2021. It is most commonly encountered in diabetic people. Immuno-suppressive medications, steroids, and plasmapheresis are all used in the treatment of CIDP. IVIG is a safe and effective alternative to these treatments. The market growth is aided by associated advantages such as safety, minimally invasive treatment, and user-friendly treatment options.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 10.9 Billion |
| Market size forecast in 2028 | USD 17.3 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 6.9% from 2022 to 2028 |
| Number of Pages | 209 |
| Number of Tables | 319 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Application, Distribution Channel, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. The Asia Pacific witnessed a promising revenue share in the intravenous immunoglobulin market in 2021. As a result of increased awareness and potential chances for the acceptance of immunoglobulin-based therapies for the treatment of primary immunological deficiencies, as well as a growing geriatric population. Emerging economies, rising healthcare spending, and the rapidly expanding immunoglobulin market are all contributing to the market growth. Moreover, the rising prevalence of immunological illnesses increased awareness of intravenous and subcutaneous immunoglobulin therapy, and improved healthcare facilities are expected to drive future growth.
Free Valuable Insights: Global Intravenous Immunoglobulin Market size to reach USD 17.3 Billion by 2028
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Grifols, S.A., Takeda Pharmaceutical Company Limited, Baxter International, Inc., Biotest AG (Tiancheng International Investment Limited), LFB S.A., CSL Limited (CSL Behring), Octapharma AG, Kedrion S.p.A, and Bayer AG.
By Distribution Channel
By Application
By Geography
The global intravenous immunoglobulin market size is expected to reach $17.3 billion by 2028.
Growth in R&D activities in Intravenous Immunoglobulin are driving the market in coming years, however, The likeliness of blood transfused diseases limited the growth of the market.
Grifols, S.A., Takeda Pharmaceutical Company Limited, Baxter International, Inc., Biotest AG (Tiancheng International Investment Limited), LFB S.A., CSL Limited (CSL Behring), Octapharma AG, Kedrion S.p.A, and Bayer AG.
The expected CAGR of the intravenous immunoglobulin market is 6.9% from 2022 to 2028.
The Hospital Pharmacy market dominated the Global Intravenous Immunoglobulin Market by Distribution Channel in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $9.03 billion by 2028.
The North America is the fastest growing region in the Global Intravenous Immunoglobulin Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $7.51 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

