Japan Mycoplasma Testing Market Size, Share & Trends Analysis Report By Product & Service (Kits & Reagents, Instruments and Services), By Technology, By Application, By End Japaner, and Forecast, 2023 - 2030
Published Date : 20-May-2024 |
Pages: 76 |
Formats: PDF |
COVID-19 Impact on the Japan Mycoplasma Testing Market
The Japan Mycoplasma Testing Market size is expected to reach $88.99 Million by 2030, rising at a market growth of 11.7% CAGR during the forecast period.
The mycoplasma testing market in Japan has witnessed significant growth in recent years, driven by the increasing awareness of the importance of mycoplasma detection in various industries, particularly in biopharmaceutical and cell culture research. One of the key factors contributing to the growth of the mycoplasma testing market in Japan is the growing biopharmaceutical industry in the country. Japan has emerged as a hub for biopharmaceutical research and development, with an increasing number of companies focusing on the development of novel biologics.
In recent years, the pharmaceuticals industry in Japan has witnessed a significant expansion, particularly in the realm of biotechnology, with a notable focus on the mycoplasma testing market. Japan's pharmaceutical sector has embraced biotechnological advancements, leveraging cutting-edge research and development to enhance drug discovery and manufacturing processes. The mycoplasma testing market plays a crucial role in this landscape, as mycoplasma contamination compromises the quality and safety of biopharmaceutical products.
According to the International Trade Administration, biopharmaceuticals constitute around 15% of total drug sales in Japan, a figure that underscores the country's current position in the global pharmaceutical landscape. Japanese companies have an industry share of only about 3% for biopharmaceutical products developed in the new modality area. This shift signifies a broader trend towards the increased prominence of biopharmaceuticals worldwide.
Increased awareness about the risks associated with mycoplasma contamination has prompted a shift in the mindset of companies and research institutions in Japan. Training programs and educational initiatives have been implemented to ensure that professionals are well-informed about the importance of mycoplasma testing. This awareness has translated into a proactive approach, with Japanese organizations taking preventive measures to safeguard their cell cultures.
The outbreak of the COVID-19 pandemic has had a mixed impact on the mycoplasma testing market in Japan. While the pandemic initially disrupted supply chains and research activities, leading to a temporary slowdown, the focus on biopharmaceutical research and development intensified in response to the global health crisis. The need for vaccine and therapeutic development prompted a renewed emphasis on maintaining the quality and reliability of research, including mycoplasma testing.
Market Trends
Adoption of advanced technologies in biotechnology
In Japan, the mycoplasma testing market has witnessed a significant evolution with the rapid adoption of advanced technologies in the field of biotechnology. One of the key factors driving the adoption of advanced technologies in mycoplasma testing is the heightened awareness among Japanese biotechnology and pharmaceutical companies regarding the potential consequences of contamination. As these industries strive to maintain high product quality standards, the demand for more sophisticated testing methodologies has surged.
Japan has seen an increased utilization of molecular biology techniques such as polymerase chain reaction (PCR) and nucleic acid amplification tests (NAATs) for mycoplasma detection in recent years. Japanese companies are investing in state-of-the-art laboratory equipment and automation to streamline testing processes, reducing turnaround times and enhancing overall efficiency. Furthermore, stringent regulatory frameworks in Japan have played a pivotal role in shaping the mycoplasma testing landscape. The Japanese regulatory authorities emphasize the importance of rigorous testing protocols to ensure the safety of biopharmaceutical products.
The Japanese government has proactively supported the biotechnology sector through initiatives encouraging innovation and technological advancements. Japan has shown a growing interest in cell and gene therapies, which are highly sensitive to mycoplasma contamination. The surge in research and development in these areas has heightened the demand for advanced testing methods. Consequently, Japanese biotechnology firms are investing in technologies that quickly and accurately detect mycoplasma to ensure the safety and efficacy of cell and gene therapy products. Hence, the mycoplasma testing market in Japan is experiencing significant advancements driven by heightened awareness, adoption of molecular biology techniques, and support from proactive government initiatives.
Increasing research and development activities
In recent years, Japan has witnessed a substantial surge in research and development activities within the mycoplasma testing market, reflecting a growing emphasis on ensuring the safety and efficacy of biopharmaceutical and biotechnology products. Mycoplasma contamination poses a significant threat to cell cultures, vaccines, and therapeutic proteins, making robust testing protocols a priority for the Japanese biopharmaceutical sector.
One key driver behind the heightened R&D focus is the increasing awareness among Japanese researchers and industry professionals about the potential risks associated with mycoplasma contamination. Japan's stringent regulatory environment also plays a crucial role in propelling research and development activities in mycoplasma testing. Regulatory bodies such as the Pharmaceuticals and Medical Devices Agency (PMDA) have underscored the importance of implementing effective quality control measures to ensure the safety of biopharmaceuticals.
Moreover, collaborations between academic institutions, research organizations, and biotechnology companies in Japan have fueled innovation in mycoplasma testing. The mycoplasma testing market in Japan is witnessing an influx of investments from domestic and international stakeholders. Japanese companies specializing in life sciences and biotechnology actively participate in research initiatives to enhance the sensitivity and specificity of mycoplasma testing assays. Thus, Japan's mycoplasma testing market has experienced significant growth driven by heightened awareness of contamination risks, stringent regulatory measures, and collaborative efforts among industry players and research institutions.
Competition Analysis
The mycoplasma testing market in Japan is characterized by a diverse range of companies that play a crucial role in ensuring the quality and safety of biological products. These companies contribute significantly to the life sciences and biotechnology sectors, supporting research and development efforts and maintaining the integrity of biological materials.
In Japan, Sysmex Corporation is a prominent player in the mycoplasma testing market. Sysmex is renowned for its innovative solutions in the field of in vitro diagnostics, offering a comprehensive range of products for hematology, urinalysis, and molecular diagnostics. The company's mycoplasma testing solutions are designed to meet the Japanese biopharmaceutical industry's stringent requirements, ensuring biological product reliability.
Another key player in the Japanese mycoplasma testing landscape is Nipro Corporation. Nipro is a leading global manufacturer of medical devices and pharmaceutical products. The company's involvement in mycoplasma testing underscores its commitment to quality control and safety in the production of biopharmaceuticals. Nipro's state-of-the-art facilities in Japan contribute to the overall infrastructure supporting mycoplasma testing in the country.
Tosoh Corporation is also a significant contributor to the mycoplasma testing market in Japan. Specializing in developing and manufacturing advanced diagnostic systems and reagents, Tosoh plays a crucial role in enhancing the accuracy and efficiency of mycoplasma detection. The company's commitment to research and development ensures that its mycoplasma testing solutions align with the evolving needs of the Japanese biopharmaceutical industry.
In addition to these major players, smaller niche companies, such as Seikagaku Corporation, contribute their expertise to the mycoplasma testing sector. Seikagaku focuses on developing high-quality reagents and analytical instruments, catering to the specific requirements of Japan's biopharmaceutical and life sciences sectors. These specialized companies often play a pivotal role in offering tailored solutions to address unique challenges in mycoplasma testing. Collectively, these companies form a robust ecosystem that upholds the highest standards in the mycoplasma testing market in Japan.
List of Key Companies Profiled
- Charles River Laboratories International, Inc.
- Thermo Fisher Scientific, Inc.
- Eurofins Scientific SE
- Lonza Group Ltd.
- Bio-Rad laboratories, Inc.
- InvivoGen SAS
- Asahi Kasei Corporation
- F.Hoffmann-La Roche Ltd.
- Norgen Biotek Corp.
- PromoCell GmbH
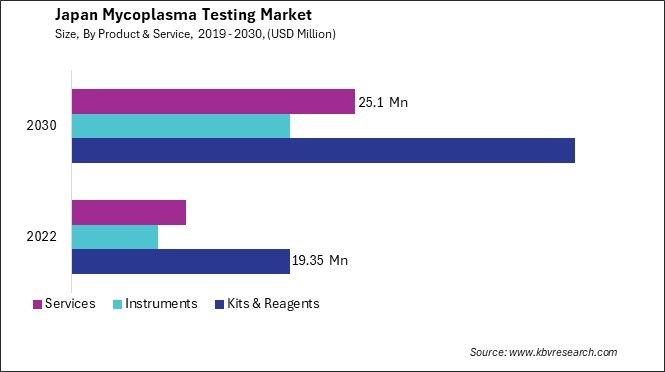
Japan Mycoplasma Testing Market Report Segmentation
By Product & Service
- Kits & Reagents
- Instruments
- Services
By Technology
- PCR
- ELISA
- Microbial Culture Techniques
- Enzymatic Methods
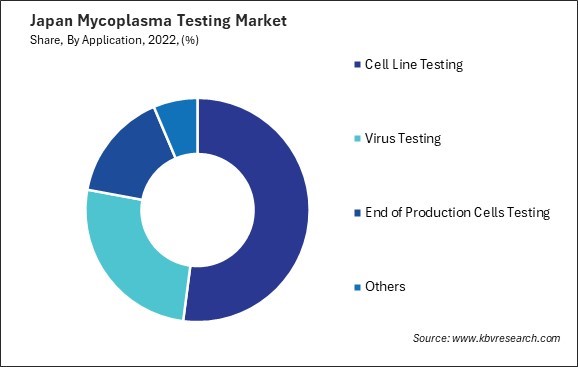
By Application
- Cell Line Testing
- Virus Testing
- End of Production Cells Testing
- Others
By End User
- Pharmaceutical & Biotechnology Companies
- Contract Research Organizations
- Academic Research Institutes
- Cell Banks
- Others
1.1 Market Definition
1.2 Objectives
1.3 Market Scope
1.4 Segmentation
1.4.1 Japan Mycoplasma Testing Market, by Product & Service
1.4.2 Japan Mycoplasma Testing Market, by Technology
1.4.3 Japan Mycoplasma Testing Market, by Application
1.4.4 Japan Mycoplasma Testing Market, by End User
1.5 Methodology for the research
Chapter 2. Market Overview
2.1 Introduction
2.1.1 Overview
2.1.1.1 Market Composition and Scenario
2.2 Key Factors Impacting the Market
2.2.1 Market Drivers
2.2.2 Market Restraints
2.2.3 Market Trends
2.3 Porter Five Forces Analysis
Chapter 3. Japan Mycoplasma Testing Market
3.1 Japan Mycoplasma Testing Market by Product & Service
3.2 Japan Mycoplasma Testing Market by Technology
3.3 Japan Mycoplasma Testing Market by Application
3.4 Japan Mycoplasma Testing Market by End User
Chapter 4. Company Profiles – Global Leaders
4.1 Charles River Laboratories International, Inc.
4.1.1 Company Overview
4.1.2 Financial Analysis
4.1.3 Segmental and Regional Analysis
4.2 Thermo Fisher Scientific, Inc.
4.2.1 Company Overview
4.2.2 Financial Analysis
4.2.3 Segmental and Regional Analysis
4.2.4 Research & Development Expenses
4.2.5 SWOT Analysis
4.3 Eurofins Scientific SE
4.3.1 Company Overview
4.3.2 Financial Analysis
4.3.3 Regional Analysis
4.3.4 Recent strategies and developments:
4.3.4.1 Product Launches and Product Expansions:
4.3.4.2 Acquisition and Mergers:
4.3.5 SWOT Analysis
4.4 Lonza Group Ltd.
4.4.1 Company Overview
4.4.2 Financial Analysis
4.4.3 Segmental and Regional Analysis
4.4.4 Research & Development Expenses
4.4.5 SWOT Analysis
4.5 Bio-Rad laboratories, Inc.
4.5.1 Company Overview
4.5.2 Financial Analysis
4.5.3 Segmental and Regional Analysis
4.5.4 Research & Development Expenses
4.5.5 SWOT Analysis
4.6 InvivoGen SAS
4.6.1 Company Overview
4.6.2 SWOT Analysis
4.7 Asahi Kasei Corporation
4.7.1 Company Overview
4.7.2 Financial Analysis
4.7.3 Segmental and Regional Analysis
4.7.4 Research & Development Expenses
4.7.5 SWOT Analysis
4.8 F. Hoffmann-La Roche Ltd.
4.8.1 Company Overview
4.8.2 Financial Analysis
4.8.3 Segmental and Regional Analysis
4.8.4 Research & Development Expense
4.8.5 SWOT Analysis
4.9 Norgen Biotek Corp.
4.9.1 Company Overview
4.10. PromoCell GmbH
4.10.1 Company Overview
4.10.2 SWOT Analysis
TABLE 2 Japan Mycoplasma Testing Market, 2023 - 2030, USD Million
TABLE 3 Japan Mycoplasma Testing Market by Product & Service, 2019 - 2022, USD Million
TABLE 4 Japan Mycoplasma Testing Market by Product & Service, 2023 - 2030, USD Million
TABLE 5 Japan Mycoplasma Testing Market by Technology, 2019 - 2022, USD Million
TABLE 6 Japan Mycoplasma Testing Market by Technology, 2023 - 2030, USD Million
TABLE 7 Japan Mycoplasma Testing Market by Application, 2019 - 2022, USD Million
TABLE 8 Japan Mycoplasma Testing Market by Application, 2023 - 2030, USD Million
TABLE 9 Japan Mycoplasma Testing Market by End User, 2019 - 2022, USD Million
TABLE 10 Japan Mycoplasma Testing Market by End User, 2023 - 2030, USD Million
TABLE 11 Key Information – Charles River Laboratories International, Inc.
TABLE 12 Key Information – Thermo Fisher Scientific, Inc.
TABLE 13 Key Information – Eurofins Scientific SE
TABLE 14 Key Information –Lonza Group Ltd.
TABLE 15 Key Information – Bio-Rad Laboratories, Inc.
TABLE 16 Key Information – InvivoGen SAS
TABLE 17 Key Information – Asahi Kasei Corporation
TABLE 18 Key Information – F. Hoffmann-La Roche Ltd.
TABLE 19 Key Information – Norgen Biotek Corp.
TABLE 20 Key Information – PromoCell GmbH
List of Figures
FIG 1 Methodology for the research
FIG 2 Japan Mycoplasma Testing Market, 2019 - 2030, USD Million
FIG 3 Key Factors Impacting Mycoplasma Testing Market
FIG 4 Porter’s Five Forces Analysis – Mycoplasma Testing Market
FIG 5 Japan Mycoplasma Testing Market share by Product & Service, 2022
FIG 6 Japan Mycoplasma Testing Market share by Product & Service, 2030
FIG 7 Japan Mycoplasma Testing Market by Product & Service, 2019 - 2030, USD Million
FIG 8 Japan Mycoplasma Testing Market share by Technology, 2022
FIG 9 Japan Mycoplasma Testing Market share by Technology, 2030
FIG 10 Japan Mycoplasma Testing Market by Technology, 2019 - 2030, USD Million
FIG 11 Japan Mycoplasma Testing Market share by Application, 2022
FIG 12 Japan Mycoplasma Testing Market share by Application, 2030
FIG 13 Japan Mycoplasma Testing Market by Application, 2019 - 2030, USD Million
FIG 14 Japan Mycoplasma Testing Market share by End User, 2022
FIG 15 Japan Mycoplasma Testing Market share by End User, 2030
FIG 16 Japan Mycoplasma Testing Market by End User, 2019 - 2030, USD Million
FIG 17 SWOT Analysis: Thermo Fisher Scientific, Inc.
FIG 18 SWOT Analysis: Eurofins Scientific SE
FIG 19 SWOT Analysis: Lonza Group Ltd.
FIG 20 SWOT Analysis: Bio-Rad Laboratories, Inc.
FIG 21 SWOT Analysis: InvivoGen SAS
FIG 22 SWOT Analysis: Asahi Kasei Corporation
FIG 23 SWOT Analysis: F. Hoffmann-La Roche Ltd.
FIG 24 SWOT Analysis: PromoCell GmbH