Int'l : +1(646) 600-5072 | query@kbvresearch.com
Int'l : +1(646) 600-5072 | query@kbvresearch.com
Published Date : 15-May-2024 |
Pages: 102 |
Formats: PDF |
The Japan Pharmaceutical Drug Delivery Market size is expected to reach $102.72 Billion by 2030, rising at a market growth of 4.3% CAGR during the forecast period.
Japan's pharmaceutical drug delivery market has witnessed significant growth and innovation, reflecting the nation's commitment to advancing healthcare solutions. Japan's aging population, technological advancements, and robust healthcare infrastructure have contributed to the flourishing drug delivery sector. Local regulatory agencies, such as the Pharmaceuticals and Medical Devices Agency (PMDA), play a crucial role in shaping the drug delivery landscape in Japan. The COVID-19 pandemic has had a significant impact on the pharmaceutical drug delivery market in Japan, prompting rapid innovation and adaptation within the industry. The urgent need for effective treatments and vaccines has accelerated research efforts towards novel drug delivery technologies for antiviral medications and vaccines.
Patient-centric approaches are gaining prominence, focusing on improving medication adherence and overall patient experience. Japan's pharmaceutical drug delivery market is characterized by a diverse range of delivery technologies catering to different therapeutic areas. Inhalation drug delivery systems, for example, have gained traction, particularly for treating respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD). Japanese companies are investing in developing advanced inhalation devices that provide precise and targeted delivery of respiratory medications.
The pharmaceutical industry in Japan has witnessed a surge in the development of biologics and specialty drugs. As these complex molecules often require specific delivery mechanisms, a growing focus is on advancing drug delivery technologies to ensure optimal therapeutic outcomes. Japanese companies are exploring innovative solutions such as nanoparticles, liposomes, and micelles to improve the delivery of biologics.
According to the International Trade Administration, Japanese pharmaceutical companies collaborate with artificial intelligence startups. The success rate of new drug development in the Japanese pharmaceutical industry is about 1 in 20,000 to 1 in 30,000, and the development period until industry launch takes over ten years. Such new drug development efforts require approximately 120 billion yen or approximately $834 million in investments. The integration of AI technologies in the pharmaceutical drug development process holds promise for streamlining research, identifying potential drug candidates more rapidly, and optimizing various stages of the development lifecycle.
The pharmaceutical drug delivery landscape has witnessed a significant transformation in Japan with the growing prominence of biologics and large molecule drugs. Biologics, which include vaccines, gene therapies, and monoclonal antibodies, have gained considerable traction in Japan's pharmaceutical drug delivery market. The unique characteristics of biologics enable them to address previously challenging medical conditions, fostering a new era of precision medicine in the country.
Large molecule drugs, characterized by their complex structures and diverse mechanisms of action, have also become integral to Japan's pharmaceutical landscape. Their ability to modulate intricate biological pathways has proven effective in treating diseases with multifaceted etiologies. The rising approvals and industry authorizations for these innovative therapies reflect the Japanese pharmaceutical industry's embrace of biologics and large molecule drugs.
Regulatory agencies in Japan have adapted to the evolving landscape by establishing frameworks that facilitate the efficient evaluation and approval of such advanced therapeutic entities. Furthermore, the prevalence of an aging population in Japan has heightened the demand for sophisticated treatment modalities. Biologics and large molecule drugs are crucial in addressing age-related diseases, offering therapeutic options beyond traditional small-molecule pharmaceuticals.
According to the International Trade Administration, the proportion of the population older than 65 will rise from 29% to 40 % by 2060. This trend reflects an evolving landscape in drug delivery strategies, indicating a growing reliance on advanced pharmaceutical technologies to cater to the healthcare needs of an aging population. Therefore, Japan's pharmaceutical drug delivery landscape has significantly shifted towards biologics and large molecule drugs, driving innovation and precision medicine.
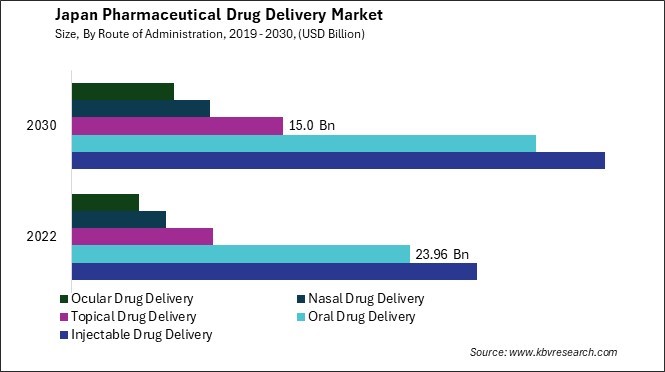
In recent years, Japan has witnessed a notable surge in adopting oral drug delivery systems within the pharmaceutical drug delivery market. One driving force behind the increased acceptance of oral drug delivery in Japan is the emphasis on patient convenience and compliance. The aging population in Japan, characterized by a significant proportion of elderly individuals, has necessitated the development of drug delivery methods that are user-friendly and easily manageable for this demographic. Oral formulations often result in cost savings for patients and the healthcare system compared to more invasive delivery methods such as injections. This cost-effectiveness is particularly relevant in Japan's healthcare system, where there is an ongoing effort to manage healthcare expenditures efficiently.
Moreover, the Japanese regulatory environment has played a pivotal role in shaping the preferences of pharmaceutical companies and healthcare providers. Regulatory authorities in Japan have been proactive in encouraging innovation and ensuring the safety and efficacy of oral drug delivery systems. This supportive regulatory framework has incentivized pharmaceutical companies in Japan to invest in research and development efforts focused on enhancing oral drug delivery technologies. Oral formulations often result in cost savings for patients and the healthcare system compared to more invasive delivery methods such as injections. This cost-effectiveness is particularly relevant in Japan's healthcare system, where there is an ongoing effort to manage healthcare expenditures efficiently.
The preference for oral drug delivery is further reinforced by cultural factors in Japan, where there is a historical inclination towards natural and holistic approaches to healthcare. The cultural acceptance of traditional herbal medicines, often administered orally, aligns with the increasing interest in oral drug delivery methods that leverage modern pharmaceutical science and traditional healing practices. Hence, the surge in oral drug delivery adoption in Japan is driven by a combination of factors, including a focus on patient convenience, cost-effectiveness, and cultural preferences for user-friendly and holistic healthcare approaches.
The pharmaceutical drug delivery market in Japan is marked by a combination of global pharmaceutical companies and local players contributing to advancing drug delivery technologies. One of the major players in the Japanese pharmaceutical drug delivery market is Takeda Pharmaceutical Company Limited. Takeda is a leading Japanese pharmaceutical company with a global presence and a strong focus on research and development. The company has been actively involved in developing advanced drug delivery systems to improve patient treatment outcomes. Takeda's efforts include exploring novel formulations, such as extended-release and controlled-release technologies, to enhance drug efficacy and patient adherence.
Astellas Pharma Inc., another key player in the Japanese industry, is known for its commitment to research and development in drug delivery. The brand creates innovative formulations and delivery methods to optimize therapeutic effects and improve patient convenience. The company has been at the forefront of developing sustained-release formulations and targeted drug delivery systems.
Local companies such as Otsuka Pharmaceutical Co., Ltd. play a pivotal role in shaping drug delivery advancements within Japan. Otsuka is recognized for its expertise in developing innovative delivery systems for various therapeutic areas, including central nervous system disorders and cardiovascular diseases. The company focuses on oral, injectable, and transdermal drug delivery technologies.
In the realm of drug delivery devices, Terumo Corporation is a key player in Japan. Terumo specializes in manufacturing medical devices, including injection and infusion systems, contributing to the safe and efficient administration of medications. The company's commitment to precision and quality aligns with the high standards of the Japanese healthcare system.
Mitsubishi Tanabe Pharma Corporation is another notable Japanese pharmaceutical drug delivery market participant. The company has developed advanced drug delivery technologies, including sustained-release formulations and innovative injection devices. Mitsubishi Tanabe's efforts aim to improve patient compliance and treatment outcomes across various therapeutic areas. As Japan continues to prioritize advancements in healthcare, these companies play a vital role in driving the evolution of drug delivery technologies to meet the specific needs of the Japanese population.
By Route of Administration
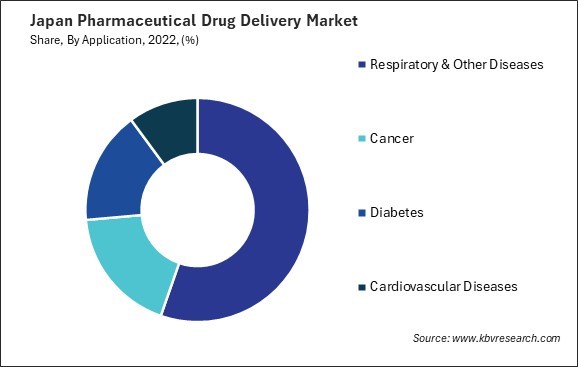
By Application