The Japan Pharmaceutical Sterility Testing Market size is expected to reach $144.9 Million by 2030, rising at a market growth of 11.1% CAGR during the forecast period.
The pharmaceutical sterility testing market in Japan is crucial to ensuring the safety and efficacy of pharmaceutical products in the country. Sterility testing plays a vital role in quality control, ensuring that pharmaceutical products are free from harmful microorganisms that pose serious patient risks. In Japan, where the pharmaceutical industry is highly regulated and quality standards are stringent, sterility testing is a mandatory requirement for all pharmaceutical products before they are released to the industry.
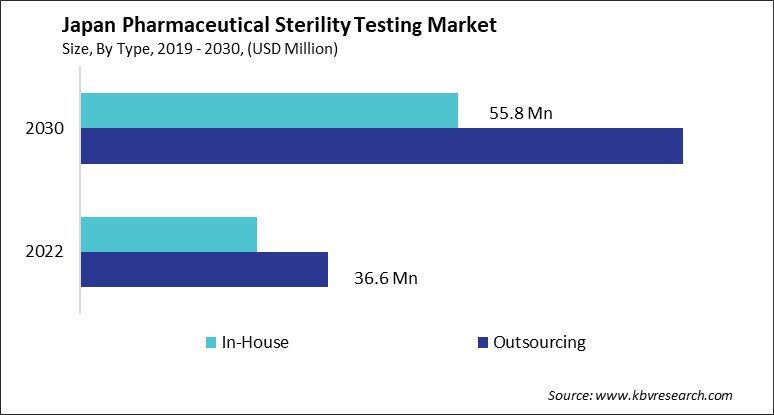
According to the Japan Ministry of Health, Labour and Welfare (MHLW), Japan's pharmaceutical sterility testing market is closely tied to the country's broader pharmaceutical industry, which boasted a total worth of $106 billion in 2021. Projections indicate that Japan's pharmaceuticals sector will experience a modest growth rate of 0.5–1.5% annually until 2027. This suggests that the demand for sterility testing within Japan's pharmaceutical industry will continue steadily alongside its overall growth trajectory over the coming years.
One of the key drivers of the pharmaceutical sterility testing market in Japan is the increasing demand for safe and effective pharmaceutical products. As the Japanese population continues to age and the prevalence of chronic diseases rises, there is a growing need for pharmaceuticals to treat a wide range of medical conditions. With patient safety being of paramount importance, pharmaceutical companies in Japan adhere to strict regulatory guidelines, including conducting thorough sterility testing to ensure the absence of harmful microorganisms.
The COVID-19 pandemic has significantly impacted the pharmaceutical sterility testing market in Japan, prompting adjustments in testing protocols, resource allocation, and regulatory guidelines. The pandemic has underscored the importance of robust sterility testing procedures in ensuring the safety and efficacy of pharmaceutical products, particularly vaccines and other critical treatments. Japan's Pharmaceutical companies have ramped up production to meet the unprecedented demand for COVID-19 therapeutics and vaccines, increasing pressure on sterility testing facilities and resources. Moreover, the pandemic has highlighted the need for rapid and scalable sterility testing solutions to support accelerated drug development timelines and emergency response efforts.
Japan's pharmaceutical sterility testing market has witnessed a significant rise in the adoption of biopharmaceuticals, reflecting a broader global trend towards these innovative therapies. Biopharmaceuticals, medicinal products manufactured using living organisms or their components, offer distinct advantages over traditional small molecule drugs, including greater specificity, reduced side effects, and enhanced efficacy. This shift towards biopharmaceuticals has reshaped the landscape of pharmaceutical development and production in Japan, driving the demand for advanced sterility testing technologies tailored to these complex formulations.
According to the International Trade Administration, the growing prevalence of biopharmaceuticals in Japan will comprise approximately 15% of total biopharmaceutical sales in 2021. This trend mirrors the increasing adoption of biopharmaceuticals in Japan's pharmaceutical sterility testing market.
Biopharmaceuticals have demonstrated remarkable efficacy in treating conditions such as cancer, autoimmune disorders, and rare genetic diseases, offering new hope for patients who have previously had limited treatment options. As a result, pharmaceutical companies in Japan are increasingly investing in the development and production of biologics, thereby fueling the demand for robust sterility testing solutions to ensure the safety and efficacy of these products.
Moreover, the Japanese regulatory environment has been conducive to the approval and commercialization of biopharmaceuticals, with regulatory agencies such as the Pharmaceuticals and Medical Devices Agency (PMDA) implementing guidelines that support developing and evaluating these innovative therapies. Furthermore, advancements in bioprocessing technologies have facilitated the production of biopharmaceuticals at larger scales, allowing for increased accessibility and affordability of these therapies in Japan. Hence, the growing adoption of biopharmaceuticals in Japan drives demand for advanced sterility testing solutions, reshaping the pharmaceutical landscape.
Japan's pharmaceutical sterility testing market is witnessing a notable surge in demand for bacterial endotoxin testing. Bacterial endotoxins, also known as pyrogens, are ubiquitous in the environment and pose significant risks to human health if present in pharmaceutical products. One of the primary drivers behind this escalating demand is the growing awareness among pharmaceutical companies regarding the importance of endotoxin testing in ensuring product quality and patient safety. With increasing regulatory scrutiny and evolving industry standards, stakeholders are prioritizing comprehensive sterility testing protocols encompassing the detection and quantification of bacterial endotoxins.
Moreover, the rise in pharmaceutical manufacturing activities in Japan and advancements in analytical techniques have facilitated more sensitive and accurate detection methods for bacterial endotoxins. Additionally, the evolving landscape of healthcare delivery in Japan, characterized by an aging population and a growing prevalence of chronic diseases, has underscored the need for stringent quality control measures in pharmaceutical production.
Furthermore, the global harmonization of regulatory guidelines and standards has prompted Japanese pharmaceutical companies to align their practices with international benchmarks, driving the widespread adoption of bacterial endotoxin testing as an integral component of sterility testing protocols. Therefore, the surge in demand for bacterial endotoxin testing in Japan's pharmaceutical sterility testing market is driven by increasing awareness, regulatory scrutiny, and technological advancements.
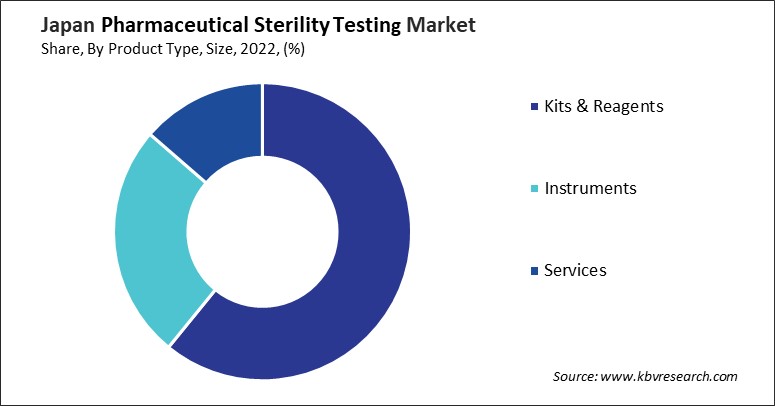
The pharmaceutical sterility testing market in Japan is vital to the global healthcare industry, ensuring that pharmaceutical products are free from harmful microorganisms and safe for human use. Japan, known for its advanced technology and strict regulatory standards, hosts several key players in this sector. One notable company in the Japanese pharmaceutical sterility testing market is Shimadzu Corporation. With a history spanning over a century, Shimadzu is a global leader in analytical and measuring instruments, including those used in sterility testing. The company offers a range of equipment and systems for microbial detection, air sampling, and environmental monitoring, essential for ensuring pharmaceutical products' sterility.
Another significant player is TOYOBO CO., LTD., which is significant in the life sciences, including sterility testing. They offer a range of biotechnology-related products and services, focusing on genetic engineering and bioprocessing. TOYOBO's contributions to the pharmaceutical sterility testing market include reagents and systems for PCR testing, which can be applied in detecting microbial contamination in pharmaceutical products.
Merck KGaA, operating under the brand name Merck Millipore in Japan, is another leading provider of solutions for pharmaceutical and biotechnology companies. Their portfolio includes sterility testing kits, microbial detection systems, and services that support the pharmaceutical industry's regulatory compliance and quality assurance needs. Merck's commitment to innovation and quality has made it a preferred partner for sterility testing solutions in Japan.
Japanese domestic firms also play a crucial role in the pharmaceutical sterility testing market. One such company is Nissui Pharmaceutical Co., Ltd., which specializes in the development, manufacture, and sale of diagnostic reagents and testing equipment for microbiological applications. Their products are widely used in pharmaceutical and food safety testing, reflecting the company's expertise in ensuring product sterility and compliance with Japan's rigorous health and safety regulations. These companies comply with Japan's stringent regulatory standards and contribute to global efforts to safeguard public health by providing sterile pharmaceuticals. With ongoing technological advancements and a collaborative industry environment, Japan's role in the pharmaceutical sterility testing market is poised to grow even further.
By Type
By Product Type
By Sample
By End Use
By Test Type
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

