The Latin America, Middle East and Africa Pharmaceutical Sterility Testing Market would witness market growth of 13.7% CAGR during the forecast period (2023-2030).
The Brazil market dominated the LAMEA Pharmaceutical Sterility Testing Market by Country in 2022 and would continue to be a dominant market till 2030; thereby, achieving a market value of $57.4 Million by 2030. The Argentina market is showcasing a CAGR of 14.3% during (2023 - 2030). Additionally, The UAE market would register a CAGR of 13.3% during (2023 - 2030).
Efforts towards regulatory harmonization are underway. Collaborative initiatives among regulatory agencies aim to standardize sterility testing requirements, reducing complexities for pharmaceutical companies operating in multiple regions and promoting consistency in quality standards. Regulatory harmonization is an ongoing process that evolves with the changing landscape of the pharmaceutical industry. As new technologies emerge and scientific understanding advances, regulatory frameworks must adapt to ensure that sterility testing standards remain robust and relevant. Continued collaboration and dialogue among regulatory agencies are essential for navigating these changes successfully.
In addition, quality by design principles are increasingly being applied to sterility testing processes. This approach emphasizes a systematic and science-based understanding of the manufacturing process, promoting the design of robust and reliable testing procedures. Traditional periodic monitoring has evolved into continuous monitoring systems, allowing real-time surveillance of environmental conditions. Automated systems with sensors constantly measure air quality, temperature, humidity, and particulate levels. This continuous approach provides a more dynamic and immediate understanding of the manufacturing environment, enabling proactive interventions.
The Saudi Arabian government has been actively investing in the healthcare sector as part of its Vision 2030 initiative, which aims to diversify the economy and improve various sectors, including healthcare. Strategic plans and investments are directed toward expanding healthcare infrastructure, enhancing medical services, and promoting research and development. The expansion of healthcare facilities and the rise in healthcare needs contribute to an increased demand for pharmaceuticals, including sterile products. Sterility testing becomes a critical step in ensuring the safety and efficacy of these pharmaceuticals before they reach the market. Thus, the rising healthcare sector in LAMEA will boost the region's demand for pharmaceutical sterility testing.
Free Valuable Insights: The Worldwide Pharmaceutical Sterility Testing Market is Projected to reach USD 3.1 Billion by 2030, at a CAGR of 10.7%
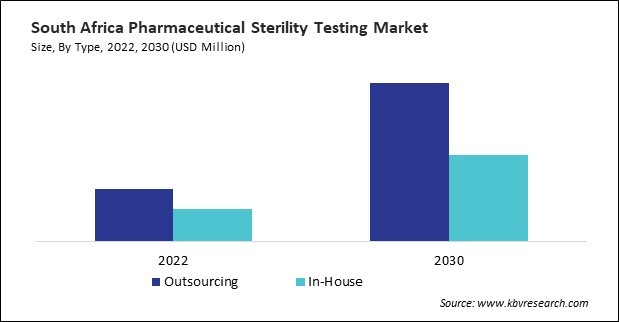
Based on Type, the market is segmented into Outsourcing, and In-House. Based on Product Type, the market is segmented into Kits & Reagents, Instruments, and Services. Based on Sample, the market is segmented into Pharmaceuticals, Medical Devices, and Biopharmaceuticals. Based on End-use, the market is segmented into Pharmaceutical Companies, Medical Device Companies, Compounding Pharmacies, and Others. Based on Test Type, the market is segmented into Bioburden Testing, Sterility Testing, and Bacterial Endotoxin Testing. Based on countries, the market is segmented into Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria, and Rest of LAMEA.
By Type
By Product Type
By Sample
By End Use
By Test Type
By Country
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

