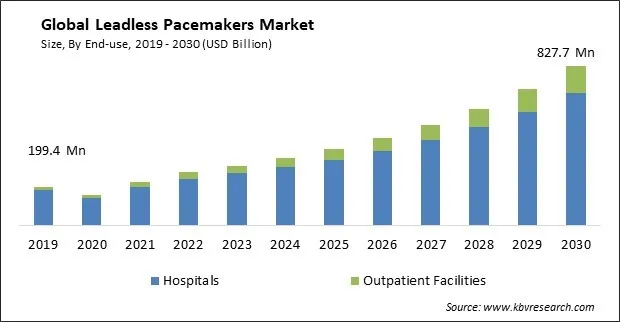
The Global Leadless Pacemakers Market size is expected to reach $827.7 million by 2030, rising at a market growth of 15.2% CAGR during the forecast period.
European patients and healthcare providers are increasingly valuing minimally invasive procedures. Thus, the European region would register nearly 28% share of the market by 2030. The catheter-based implantation of leadless pacemakers eliminates the need for surgical pockets and leads, reducing pain, scarring, and recovery time. Europe possesses a well-developed healthcare infrastructure with advanced medical facilities and resources. Many outpatient facilities as well as hospitals in this region are equipped to perform leadless pacemaker implantations and provide necessary follow-up care.
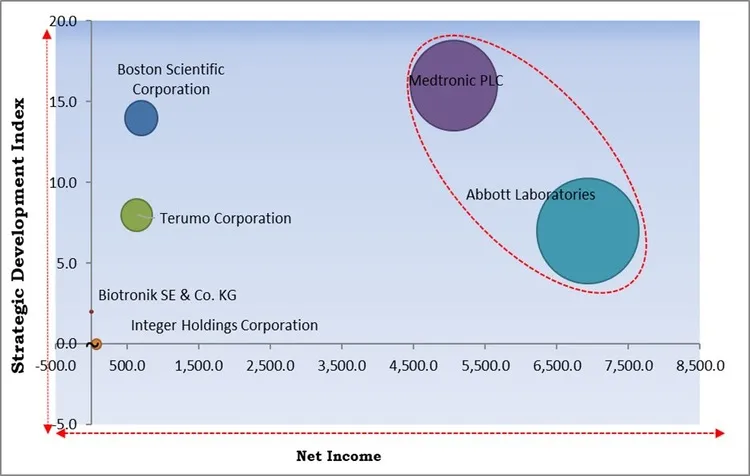
The major strategies followed by the market participants are Mergers & Acquisitionas the key developmental strategy to keep pace with the changing demands of end users. For instance, In August, 2022, Medtronic plc completed the acquisition of Affera, Inc., expanding its cardiac ablation portfolio with the addition of a groundbreaking cardiac mapping and navigation platform, featuring a fully integrated diagnostic, focal pulsed field, and radiofrequency ablation solution. Moreover, In April, 2023, Abbott Laboratories completed the acquisition of Cardiovascular Systems, Inc. (CSI), a medical device company known for its innovative atherectomy system in the treatment of peripheral and coronary artery disease. This acquisition enhances Abbott's offerings for vascular disease treatment, incorporating CSI's leading atherectomy system, which prepares vessels for angioplasty or stenting to restore blood flow.
Based on the Analysis presented in the KBV Cardinal matrix; Medtronic PLC and Abbott Laboratories are the forerunners in the Market. Companies such as Boston Scientific Corporation, Integer Holdings Corporation and Terumo Corporation are some of the key innovators in the Market. In September, 2023, Boston Scientific Corporation announced the acquisition of Relievant Medsystems, Inc., a privately held medical device company specializing in vertebrogenic pain treatment. This acquisition enables the integration of Relievant Medsystems' clinically backed technology with Boston Scientific's strategies, expanding treatment options for individuals in need of personalized care.
During the pandemic, many hospitals and healthcare facilities postponed or canceled elective procedures to free up resources and reduce the risk of virus transmission. This led to a decrease in leadless pacemaker implantations, as these procedures are often considered elective and non-urgent. The global supply chain for medical devices, including leadless pacemakers, experienced disruptions due to lockdowns and restrictions on manufacturing and transportation. This led to delays in the availability of devices and potentially impacted patients' access to these advanced technologies. Hospitals and healthcare providers redirected resources and staff to handle the surge of COVID-19 cases. This diversion of attention and resources from non-COVID medical services affected the promotion and implantation of leadless pacemakers.
The development of minimally invasive procedures has decreased the need for lead placement and surgical incisions, lowering surgical risks and enhancing patient comfort. This reduction in invasiveness has not only translated into enhanced patient safety but also quicker recovery times. Patients undergoing leadless pacemaker implantation experience shorter hospital stays and reduced post-operative discomfort. This quick recovery benefits patients by allowing them to return to their daily routines and activities more swiftly. These procedures have opened up new possibilities for remote and rural healthcare settings, where access to healthcare facilities may be limited. As a result, the rapidly increasing demand for minimally invasive procedures will aid in the expansion of the market.
The rising prevalence of cardiac arrhythmias is a complex interplay of multiple factors, from demographic changes to lifestyle choices and advances in healthcare. High blood pressure is a well-established risk factor for arrhythmias. It can lead to changes in the heart's structure and electrical signaling, increasing the likelihood of arrhythmias. Similarly, diabetes is associated with an increased risk of arrhythmias, particularly atrial fibrillation. Furthermore, the prevalence of arrhythmias is not limited to specific age groups; it affects individuals across various demographics. Patients of all ages who experience cardiac arrhythmias, from the elderly to younger individuals, can benefit from these devices. Therefore, the rising prevalence of arrhythmias is boosting the demand for leadless pacemakers.
Regulatory challenges, such as the complex and lengthy approval process for medical devices, can significantly delay the introduction of leadless pacemakers. Regulatory agencies require thorough evaluations of safety and efficacy, leading to prolonged timeframes for product clearance. Delays in regulatory approval not only hinder patients' access to advanced medical solutions but also increase the time-to-market for manufacturers, impacting their ability to offer cutting-edge devices. The economic burden of these devices can deter both healthcare providers and patients from choosing leadless pacemakers as a pacing solution despite their potential benefits. Such financial burdens may discourage some individuals from opting for these devices, especially if more cost-effective alternatives are available. All these factors may hamper the growth of the market.
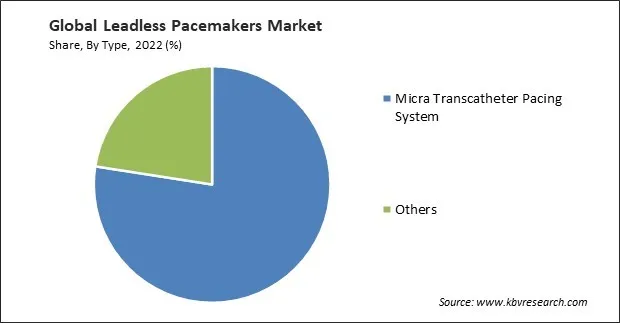
Based on type, the market is bifurcated into micra transcatheter pacing system and others. The other segment procured a considerable growth rate in the market in 2022. Leadless pacemakers, including devices like Nanostim and WiSE CRT System, offer several benefits compared to traditional pacemakers with leads. Nanostim is remarkably compact, similar in size to a large vitamin capsule. This small size allows for a less invasive procedure and results in a more unobtrusive and comfortable implantation. The WiSE CRT System is designed for cardiac resynchronization therapy, a treatment for heart failure. It offers the benefits of cardiac resynchronization without the need for traditional pacing leads. Both Nanostim and the WiSE CRT System provide significant advantages, such as minimally invasive implantation, compact designs, reduced infection risk, cosmetic benefits, and long-lasting battery life.
On the basis of end-use, the market is divided into hospitals and outpatient facilities. The hospitals segment acquired the largest revenue share in the market in 2022. Hospitals can efficiently allocate resources, as there is no need for specialized surgical suites or surgical teams dedicated to pacemaker implantations. This can lead to cost savings and the reallocation of resources to other critical medical procedures. With no leads that exit the body, the risk of infections related to lead entry sites is virtually eliminated. Hospitals can benefit from reduced rates of device-related infections.
| Report Attribute | Details |
|---|---|
| Market size value in 2022 | USD 274 Million |
| Market size forecast in 2030 | USD 827.7 Million |
| Base Year | 2022 |
| Historical Period | 2019 to 2021 |
| Forecast Period | 2023 to 2030 |
| Revenue Growth Rate | CAGR of 15.2% from 2023 to 2030 |
| Number of Pages | 199 |
| Number of Table | 244 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Type, End-use, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Region-wise, the market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America segment witnessed the maximum revenue share in the market in 2022. The demand for leadless pacemakers is steadily increasing in North America due to several factors contributing to their growing adoption in the region. These factors highlight the evolving landscape of cardiac pacing technology and the shift towards more patient-centric, minimally invasive, and technologically advanced options for treating cardiac arrhythmias. Leadless pacemakers eliminate the need for leads that can be susceptible to infection. In regions like North America, where infection control is a priority, this feature is highly valued.
Free Valuable Insights: Global Leadless Pacemakers Market size to reach USD 827.7 Million by 2030
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Medtronic PLC, Abbott Laboratories, Boston Scientific Corporation, Biotronik SE & Co. KG, Lepu Medical Technology Co., Ltd, Integer Holdings Corporation, MicroPort Scientific Corporation, Osypka AG, Terumo Corporation, and EBR Systems, Inc.
By Type
By End-use
By Geography
The Market size is projected to reach USD 827.7 million by 2030.
Growing demand for minimally invasive procedures are driving the Market in coming years, however, Regulatory and reimbursement challenges restraints the growth of the Market.
Medtronic PLC, Abbott Laboratories, Boston Scientific Corporation, Biotronik SE & Co. KG, Lepu Medical Technology Co., Ltd, Integer Holdings Corporation, MicroPort Scientific Corporation, Osypka AG, Terumo Corporation, and EBR Systems, Inc.
The expected CAGR of this Market is 15.2% from 2023 to 2030.
The Micra Transcatheter Pacing System segment is leading the Market, By Type in 2022 thereby achieving a market value of $612.9 million by 2030.
The North America region dominated the Market, By Region in 2022, and would continue to be a dominant market till 2030; thereby, achieving a market value of $384.3 million by 2030.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

