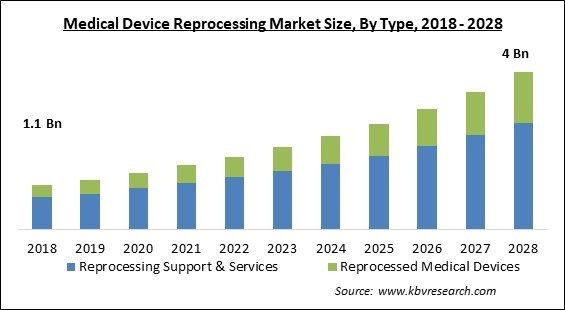
The Global Medical Device Reprocessing Market size is expected to reach $4 billion by 2028, rising at a market growth of 13.7% CAGR during the forecast period.
Cleaning, sterilizing, refurbishing, repairing, disinfecting, as well as reconditioning discarded medical devices in order to make prepare them for reuse is known as medical device reprocessing. In the healthcare business, medical devices are utilized in a number of processes, including diagnosis, monitoring, treatment, and enhancing the health and wellbeing of monitored individuals. The treatment of medical devices or their components and parts in the market for medical device reprocessing includes making them reusable.
Pre-soaking, automatic washing, manual cleaning, and disinfection are among the procedures used in the reprocessing of medical devices. Medical instruments that can be reused include stethoscopes, forceps, and endoscopes. Reprocessing may involve point-of-use treatment, packaging, cleaning, high-level disinfection, and sterilization, among other procedures, depending on the categorization of the device.
Sterilization and disinfection are crucial for preventing the transmission of infectious germs to patients through medical devices and instruments. The risk of infection is decreased and patient outcomes are enhanced in a healthcare facility by adhering to industry standards and best practices for medical device reprocessing. In addition to the Instructions for Use provided by the device's manufacturer and the rules and procedures of the healthcare facility, industry governing bodies also provide guidelines for the reprocessing of medical devices.
A sample system for classifying medical equipment into reprocessing criticality categories based on use risk is the Spaulding classification. It can be used to assist determine how much processing an instrument requires. Reprocessing used medical equipment properly is a crucial step in ensuring the safety of patients. Reprocessing is done to make sure that equipment is clean and safe for the following patient by removing tissue, blood, and other biological debris as well as by inactivating infectious bacteria.
Hospitals produce a lot of waste per bed each day, and the beginning of COVID-19 has raised this volume even more by boosting the demand for single-use medical equipment. The public, patients, healthcare staff, and waste management personnel are all at risk for health issues as a result of improper management of disposing of medical waste. By reprocessing the discarded single-use medical equipment, many medical device reprocessing service providers are implementing sustainability solutions to help manage this problem. hence enhancing waste reduction and increasing product longevity. Hence, the COVID-19 pandemic spurred the growth of the COVID-19 pandemic.
Activities related to healthcare produce a considerable amount of waste that could have a negative impact on the health of people as well as sanitary staff. The majority of this waste is not any riskier than standard home waste. Some forms of medical waste, however, pose a higher risk to health. It includes infectious waste, of which sharps trash, body part waste, pharmaceutical or chemical waste, radioactive and cytotoxic waste, and broken thermometers are included. In addition, hazardous biological waste is produced in part by medical waste. The generation and disposal of medical waste is a crucial issue, particularly in nations with a high population along with inadequate sanitation.
Tools and devices used in hospitals and other healthcare facilities are very expensive. As the number of patients needing various surgeries is increasing, affording these devices can become a significant challenge for hospitals. However, hospital sterilizing practices had to evolve as plastic materials became more prevalent. They developed in complexity and emphasized security. Nowadays, reprocessing is considered a third-party procedure. Hospitals should expect cost reductions from reprocessing companies, which also offer to contribute to their financial success.
The challenges of performing adequate cleaning, disinfection, and sterilization have grown as medical devices have gotten more complicated over time. The sterilization, disinfection, and cleaning of medical devices involve numerous stakeholders, including regulatory organizations, such as the FDA, CDC, state departments of public health, medical device makers, re-processors, standards-setting bodies, and test labs. The burden of ensuring that reprocessing methods are effective and adhered to falls on the numerous stakeholders involved in this issue.
Based on Type, the Medical Device Reprocessing Market is bifurcated into Reprocessing Support & Services and Reprocessed medical devices. In 2021, the reprocessing support & services segment held the biggest revenue share of the medical device reprocessing market. The significantly increasing growth of the market is primarily attributed to the expanding demand for ways to cut down on medical waste. In addition, the growth of the segment is also owing to an increasing number of businesses and organizations offering reprocessing services and support.
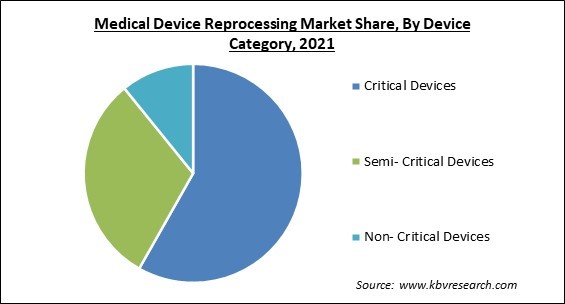
On the basis of Device Category, the Medical Device Reprocessing Market is segregated into Critical Devices, Semi- Critical Devices, and Non- Critical Devices. In 2021, the semi-critical devices segment recorded a substantial revenue share of the medical device reprocessing market. The growth of the segment is rising as a result of the increasing significance of such devices all over the world. During a process, semi-critical equipment may come into contact with mucous membranes or skin that is not intact.
By Application, the Medical Device Reprocessing Market is segmented into Cardiology, Gastroenterology, Gynecology, Arthroscopy & Orthopedic Surgery, General Surgery and Anesthesia, and Other Applications. In 2021, the gastroenterology segment garnered a significant revenue share of the medical device reprocessing market. The rising growth of the segment is majorly attributed to the rapidly changing lifestyle of people all over the world. Due to unhealthy eating patterns along with a sedentary lifestyle of people, the demand for gastroenterology treatment is inclining all over the world.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 1.6 Billion |
| Market size forecast in 2028 | USD 4 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 13.7% from 2022 to 2028 |
| Number of Pages | 230 |
| Number of Tables | 370 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Type, Device Category, Application, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Australia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Region-Wise, the Medical Device Reprocessing Market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. In 2021, North America held the largest revenue share of the medical device reprocessing market. The regional market is expanding as a result of factors, like the growing demand to lower healthcare expenses in the US and the existence of top service providers of medical device reprocessing in the US area. Moreover, the growth of the market in the region can also be linked to the expanding economy, the rise in chronic disease prevalence, and the growing demand for hospital cost savings.
Free Valuable Insights: Global Medical Device Reprocessing Market size to reach USD 4 Billion by 2028
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Stryker Corporation, Johnson & Johnson, Medline Industries, Inc. (Centurion Medical Products Corporation), Medtronic PLC, Steris Healthcare, Arjo Group (ReNu Medical, Inc.), Cardinal Health, Inc. (Sustainable Technologies), Avante Health Solutions, Konoike Group, and Vanguard AG.
By Type
By Device Category
By Application
By Geography
The Medical Device Reprocessing Market size is projected to reach USD 4 billion by 2028.
Helps Is Addressing The Issue Of Increasing Medical Waste are driving the market in coming years, however, Rising Number Of Complexities In Medical Devices restraints the growth of the market.
Stryker Corporation, Johnson & Johnson, Medline Industries, Inc. (Centurion Medical Products Corporation), Medtronic PLC, Steris Healthcare, Arjo Group (ReNu Medical, Inc.), Cardinal Health, Inc. (Sustainable Technologies), Avante Health Solutions, Konoike Group, and Vanguard AG.
The expected CAGR of the Medical Device Reprocessing Market is 13.7% from 2022 to 2028.
The Critical Devices segment acquired maximum revenue share in the Global Medical Device Reprocessing Market by Device Category in 2021 thereby, achieving a market value of $2.3 billion by 2028.
The North America market dominated the Global Medical Device Reprocessing Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $1.5 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

