The Global Minimal Residual Disease Testing Market size is expected to reach $2.7 billion by 2028, rising at a market growth of 14.2% CAGR during the forecast period.
Minimal residual disease (MRD) testing is performed to identify solid tumors or hematological malignancies. MRD tests are mostly utilized for the diagnosis of a residual diagnosis in research and clinical settings. Through this test, the oncologists receive individualized information on the effectiveness of the utilized therapy from the ongoing monitoring of leftover cancer cells during the core therapy and throughout the remission stage.
Consequently, MRD tests also affect the decision-making for the selection of efficient treatment that would result in the improvement of the therapeutic results. Additionally, these tests can accurately forecast the likelihood of potential disease relapse. The onset of hematological malignancies or blood cancers takes place in the bone marrow, where stem cells give rise to red blood cells (RBC), white blood cells (WBC), and platelets.
These diseases affect the proper generation and operation of blood cells. These malignancies form when the development of healthy blood cells is obstructed by the unchecked proliferation of abnormal cells, which interferes with the normal functions of healthy cells. MRD is also known as measurable residual testing, and its test methods are used to determine the efficacy of cancer treatment and the need to direct future treatment strategies.
Leukemia, lymphoma, and myeloma are the three blood malignancies for which MRD testing is most commonly utilized, while its usage for other diseases’ diagnoses is being researched. In addition, since the outcomes can be utilized to customize a patient’s therapy, these tests are often considered helpful in the development of customized medicine.
The modifications to treatment occurred at a considerably slow pace owing to the pandemic’s urgency. As a result, the setup of optimal time points to assess the disease response and evaluate for relapse by measuring the minimal residual disease for the newer methods could not be adequately established. This further made the intensification of therapy challenging by not providing an apt description of the therapy’s duration and time points. Therefore, the COVID-19 pandemic had a negative effect on the minimal residual disease testing market.
Since the past few decades, the incidences of hematological malignancies have risen significantly. This increase includes the rising prevalence of hematological cancer subtypes as well. For example, leukemia was the eleventh leading mortality-causing factor that was associated with cancer in 2018. According to the Surveillance, Epidemiology, and End Results (SEER) Program, the disease also accounted for around 3.2% of all new emerging cancer cases in 2022.
Since their development, minimal residual disease testing methods have become an essential part of the diagnosis of various blood cancers type. In acute lymphocytic leukemia (ALL), MRD tests are an integral part of routine testing. Several studies have also suggested that MRD tests are the most appropriate way to determine the appropriate treatment that would be extremely helpful for the first ALL therapy. These tests also help in the identification of patients that are at risk of relapse. Such factors aid in the expansion of the minimal residual disease testing market significantly.
Like any other treatment and diagnostic methods, the minimal residual disease testing methodologies are also subject to specific issues related to the usage and suitability of these tests. The issue varies according to the tests. For example, multiparameter flow cytometry tests include complex data visualization, and these tests are unable to detect cytogenetic characteristics. Therefore, the strict regulations, along with the drawbacks of MRD tests, hampers the expansion and growth of the minimal residual disease testing market.
Based on technology, the minimal residual disease testing market is categorized into flow cytometry, polymerase chain reaction (PCR), next-generation sequencing (NGS), and other technologies. The polymerase chain reaction (PCR) segment garnered the highest revenue share in the minimal residual disease testing market in 2021. The growth of the segment is because of the high practicality and simplicity of the PCR diagnostic devices in minimal residual disease testing. In addition, the PCR diagnostic device kits offer increased accessibility to information with a higher degree of sensitivity.
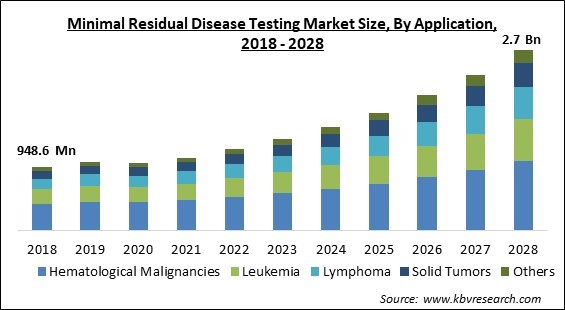
On the basis of application, the minimal residual disease testing market is divided into hematological malignancies, leukemia, lymphoma, solid tumors, and other applications. The hematological malignancies segment acquired the largest revenue share in the minimal residual disease testing market in 2021. Cancers that start forming in blood-forming tissue like bone marrow are called hematological malignancies. Lymphoma, multiple myeloma, and leukemia are all types of hematological malignancies. MRD testing using PCR diagnosis enable the identification of cancer cells, including the rare kinds which are detectable in peripheral blood or bone marrow.
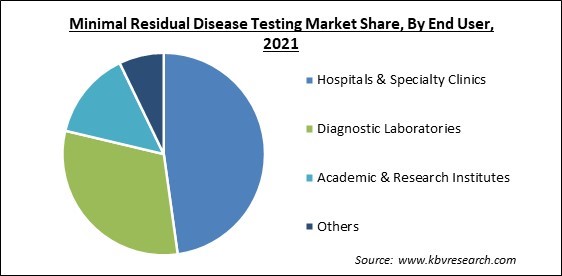
Based on end user, the minimal residual disease testing market is segmented into hospitals & specialty clinics, diagnostic laboratories, academic & research institutions, and others. The academic and research institutions segment garnered a remarkable growth rate in the minimal residual disease testing market in 2021. In academic and research institutions, the MRD test enables the conduction of more studies on rare cancer subjects. In addition, the results of MRD tests can be included as an endpoint in reports focusing on outcomes of clinical trials, which increases their use in clinical practices as well.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 1.1 Billion |
| Market size forecast in 2028 | USD 2.7 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 14.2% from 2022 to 2028 |
| Number of Pages | 246 |
| Number of Tables | 390 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Technology, Application, End User, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
On the basis of region, the minimal residual disease testing market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America segment procured the highest revenue share in the minimal residual disease testing market in 2021. MRD testing in North America is developing as a result of quick technological advancements, rising R&D expenditures, a developing patent pool along with an increase in applications.
Free Valuable Insights: Global Minimal Residual Disease Testing Market size to reach USD 2.7 Billion by 2028
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Bio-Rad Laboratories, Inc., Guardant Health, Inc., Invitae Corporation (ArcherDX, Inc.), Bio-Techne Corporation, F. Hoffmann-La Roche Ltd., ICON plc (MolecularMD Corporation), Natera, Inc., Sysmex Corporation, Laboratory Corporation of America Holdings, and Invivoscribe, Inc.
By Application
By End User
By Technology
By Geography
The global Minimal Residual Disease Market size is expected to reach $2.7 billion by 2028.
Increasing Cases Of Hematological Malignancies Across The World are driving the market in coming years, however, Complex And Strict Regulations Regarding The Application Of MRD Tests restraints the growth of the market.
Bio-Rad Laboratories, Inc., Guardant Health, Inc., Invitae Corporation (ArcherDX, Inc.), Bio-Techne Corporation, F. Hoffmann-La Roche Ltd., ICON plc (MolecularMD Corporation), Natera, Inc., Sysmex Corporation, Laboratory Corporation of America Holdings, and Invivoscribe, Inc.
The expected CAGR of the Minimal Residual Disease Market is 14.2% from 2022 to 2028.
The Hospitals & Specialty Clinics segment is generating highest revenue share in the Global Minimal Residual Disease Testing Market by End User in 2021 thereby, achieving a market value of $1.3 billion by 2028.
The North America market dominated the Global Minimal Residual Disease Testing Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $1 billion by 2028.The Europe market is experiencing a CAGR of 14% during (2022 - 2028).
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

