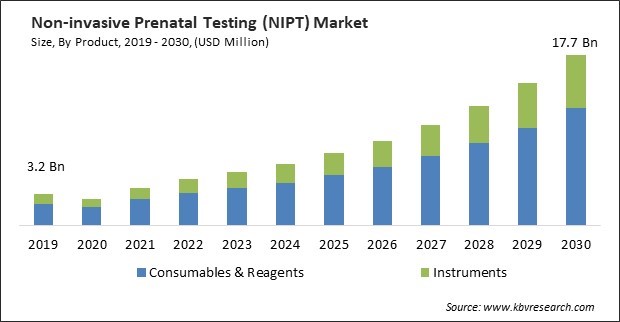
The Global Non-invasive Prenatal Testing (NIPT) Market size is expected to reach $17.7 billion by 2030, rising at a market growth of 18.1% CAGR during the forecast period.
Continuous research and development efforts have led to enhancements in the sensitivity and specificity of NIPT. This means NIPT can detect genetic abnormalities with even greater accuracy, reducing the risk of false negatives and positives. Therefore, the Next Generation Sequencing (NGS) segment captured $ 2,897.6 million revenue in the market in 2022. The improved performance is a significant driver for the adoption of NIPT. Advances in genetics and genomics have expanded the range of conditions NIPT can detect. Initially designed to identify common chromosomal abnormalities, NIPT can now detect a broader spectrum of genetic conditions, including rare genetic disorders and microdeletions. This expanded capability makes NIPT more comprehensive and attractive to expectant parents. Some of the factors impacting the market are high accuracy in detecting fetal chromosomal abnormalities, increasing awareness of their benefits, and high cost of these procedures.
The high accuracy and low false-positive rate of NIPT results instill trust and confidence in expectant parents and healthcare providers. When individuals receive NIPT results, they can be more confident in the information provided. This trust is a vital factor that positively impacts the market, as it encourages more individuals to choose NIPT over traditional screening methods. Expectant parents seek the most accurate and reliable information about their baby's health. Additionally, greater awareness and demand for NIPT naturally expands the market size. As more expectant parents seek out this non-invasive testing option, the customer base for NIPT providers grows. This leads to a larger market share and, potentially, increased revenue for NIPT companies. The demand for NIPT drives competition among providers, fostering innovation. Owing to these factors, the market will grow rapidly in the upcoming years.
However, the high cost of NIPT can limit its adoption among a significant portion of the population, particularly lower-income individuals and families. As a result, the market's potential for growth is constrained because a substantial market segment is unable to afford the testing. The cost disparity between NIPT and traditional screening methods can exacerbate healthcare inequities. Individuals without the financial means or adequate insurance coverage may be deprived of the benefits of advanced prenatal testing. This results in unequal access to superior healthcare, raising ethical and equity concerns. Thus, these factors can impede the future growth of the market.
Moreover, the pandemic led to a disruption in routine prenatal care visits. Many pregnant individuals delayed or canceled appointments to avoid the risk of contracting the virus. This impacted the timing and uptake of NIPT, as the recommended gestational age for testing is crucial for accurate results. Healthcare facilities were forced to reallocate resources to manage COVID-19 patients, which in some cases affected the availability and scheduling of prenatal care services, including NIPT. Many pregnant individuals turned to telehealth for consultations, reducing their in-person visits to healthcare facilities. NIPT providers began to explore telehealth options for consultations and result delivery to adapt to changing patient preferences. Therefore, the COVID-19 pandemic disrupted various aspects of the market.
Based on product, the market is bifurcated into consumables & reagents and instruments. In 2022, the instruments segment garnered a significant revenue share in the market. One of the primary drivers of the instruments segment's growth is the continuous evolution and improvement in NIPT technology. Advances in sequencing technologies, automation, and data analysis have led to more accurate and efficient NIPT instruments. As these technologies mature and become more accessible, the demand for upgraded instruments increases. Therefore, there will be increased demand in the instruments segment.
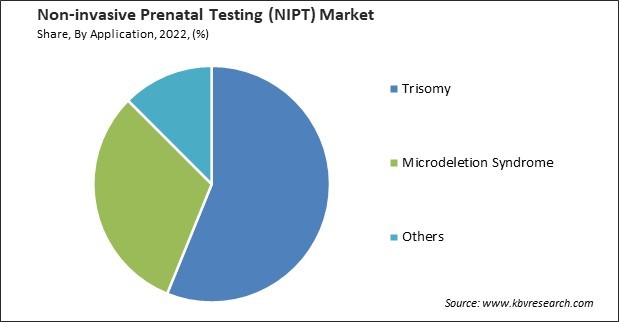
On the basis of application, the market is divided into trisomy, microdeletion syndrome, and others. The trisomy segment garnered the highest revenue share in the market in 2022. NIPT allows for the early detection of Trisomy conditions, typically as early as the 10th week of pregnancy. This early detection provides ample time for expectant parents to consider their options, make informed decisions, and plan for the future. As a result, there is no need for invasive procedures such as amniocentesis or chorionic villus sampling (CVS), which carry a small risk of complications and miscarriage.
Based on end user, the market is divided into diagnostic laboratories and hospitals & clinics. In 2022, the hospitals and clinics segment witnessed a substantial revenue share in the market. Hospitals are pivotal in providing comprehensive prenatal care to expectant mothers. This care encompasses various aspects, including regular check-ups, ultrasounds, and prenatal testing. The demand for NIPT is rising as it offers a non-invasive and accurate method for screening genetic abnormalities and chromosomal disorders, and hospitals are well-equipped to offer these services.
On the basis of technology, the market is divided into next-generation sequencing (NGS), microarray, polymerase chain reaction (PCR), and rolling circular amplification. In 2022, the rolling circular amplification segment witnessed a substantial revenue share in the market. Rolling circular amplification (RCA) technology offers improved sensitivity and specificity in detecting fetal genetic abnormalities. This is especially important for NIPT, as accurate results are crucial for making informed decisions about pregnancy management. RCA enables the detection of rare genetic variants, which traditional NIPT methods may miss. This expanded capability increases the diagnostic power of NIPT, making it a preferred choice for expectant parents seeking comprehensive genetic information.
| Report Attribute | Details |
|---|---|
| Market size value in 2022 | USD 4.8 Billion |
| Market size forecast in 2030 | USD 17.7 Billion |
| Base Year | 2022 |
| Historical Period | 2019 to 2021 |
| Forecast Period | 2023 to 2030 |
| Revenue Growth Rate | CAGR of 18.1% from 2023 to 2030 |
| Number of Pages | 300 |
| Number of Table | 430 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Product, Technology, Application, End User, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
By region, the market is segmented into North America, Europe, Asia Pacific, and LAMEA. The North America segment procured the highest revenue share in the market in 2022. North America has been at the forefront of adopting and advancing genetic sequencing technologies. The region has access to state-of-the-art sequencing platforms, which have improved the accuracy and precision of NIPT. Integrating artificial intelligence (AI) and advanced bioinformatics in data analysis has enhanced the sensitivity and specificity of NIPT, making it more reliable and attractive for both healthcare providers and expectant parents. Owing to these factors, the demand will rise in the North America segment.
Free Valuable Insights: The Global Non-invasive Prenatal Testing (NIPT) Market size to reach USD 17.7 Billion by 2030
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Illumina, Inc., Natera Inc., F. Hoffmann-La Roche Ltd., PerkinElmer, Inc., Laboratory Corporation of America Holdings, Eurofins Scientific SE, Thermo Fisher Scientific, Inc., GE HealthCare Technologies, Inc., Agilent Technologies, Inc., and Pacific Biosciences of California, Inc.
By Product
By Application
By End User
By Technology
By Geography
This Market size is expected to reach $17.7 billion by 2030.
High accuracy in detecting fetal chromosomal abnormalities are driving the Market in coming years, however, High cost of non-invasive prenatal testing procedures restraints the growth of the Market.
Illumina, Inc., Natera Inc., F. Hoffmann-La Roche Ltd., PerkinElmer, Inc., Laboratory Corporation of America Holdings, Eurofins Scientific SE, Thermo Fisher Scientific, Inc., GE HealthCare Technologies, Inc., Agilent Technologies, Inc., and Pacific Biosciences of California, Inc.
The expected CAGR of this Market is 18.1% from 2023 to 2030.
The Diagnostic Laboratories segment is registering maximum revenue in the Market by End User in 2022; thereby achieving a market value of $10.6 billion by 2030.
The North America region is generating the highest revenue in the Market by Region in 2022, and would continue to be a dominant market till 2030; thereby, achieving a market value of $7.2 billion by 2030.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

