The North America Rapid Microbiology Testing Market would witness market growth of 9.8% CAGR during the forecast period (2024-2031).
The US market dominated the North America Rapid Microbiology Testing Market by Country in 2023, and would continue to be a dominant market till 2031; thereby, achieving a market value of $2,679.3 million by 2031. The Canada market is experiencing a CAGR of 12.3% during (2024 - 2031). Additionally, The Mexico market would exhibit a CAGR of 11.3% during (2024 - 2031).
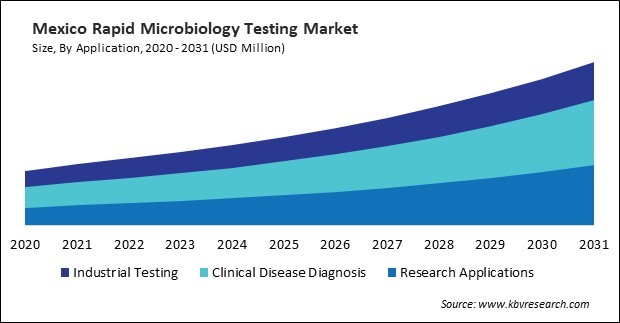
Adopting these testing technologies has gained momentum across diverse healthcare settings, including hospitals, clinics, diagnostic laboratories, and point-of-care facilities. Healthcare providers increasingly recognize the value proposition of rapid testing in facilitating prompt diagnosis, guiding targeted therapy, and minimizing the spread of infectious diseases. These testing enables healthcare professionals to make timely clinical decisions, optimize antimicrobial therapy, and improve patient outcomes by reducing turnaround times and enhancing diagnostic accuracy.
Furthermore, this testing is instrumental in antimicrobial stewardship programs to optimize antimicrobial prescribing practices, reduce unnecessary antibiotic use, and combat antimicrobial resistance. By providing rapid susceptibility testing results, rapid testing technologies enable healthcare providers to tailor antibiotic therapy to the individual patient, select the most effective antimicrobial agents, and minimize the risk of treatment failure and antimicrobial resistance development. This proactive approach to antimicrobial stewardship helps conserve antibiotics, preserve their effectiveness, and safeguard patient safety.
The Environmental Protection Agency and other regulatory agencies in the United States mandate water quality monitoring and assessment to ensure the protection of human health and the environment. In addition, the cosmetics industry in Canada is subject to stringent regulatory standards and requirements set forth by Health Canada, the regulatory authority responsible for overseeing the safety and labeling of cosmetics marketed in the country. Adherence to regulatory requirements, including Good Manufacturing Practices (GMP) and the Cosmetic Regulations, is critical in guaranteeing cosmetic products' safety, effectiveness, and quality. As per the data provided by the International Trade Administration in 2021, the cosmetics sector in Canada generated approximately USD 1.24 billion in revenue. Industry revenue is expected to reach USD 1.8 billion by 2024. Hence, North America's rising cosmetics sector and environment testing will boost the demand for this testing in the upcoming years.
Free Valuable Insights: The Rapid Microbiology Testing Market is Predict to reach USD 10.4 Billion by 2031, at a CAGR of 10.5%
Based on Method, the market is segmented into Growth-based Rapid Microbiology Testing, Cellular Component-based Rapid Microbiology Testing, Nucleic Acid-based Rapid Microbiology Testing, Viability-based Rapid Microbiology Testing and Others. Based on Application, the market is segmented into Industrial Testing (Food & Beverage Testing, Pharmaceutical & Biological Drug Testing, Environmental Testing, Cosmetics & Personal Care Products Testing and Others), Clinical Disease Diagnosis and Research Applications. Based on Product, the market is segmented into Instruments (Automated Microbial Identification & Antimicrobial Susceptibility Testing Systems, Mass Spectrometers, PCR Systems, Bioluminescence & Fluorescence-based Detection Systems, Cytometers, Active Air Samplers and Others), Reagents & Kits, and Consumables. Based on countries, the market is segmented into U.S., Mexico, Canada, and Rest of North America.
By Method
By Application
By Product
By Country
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

