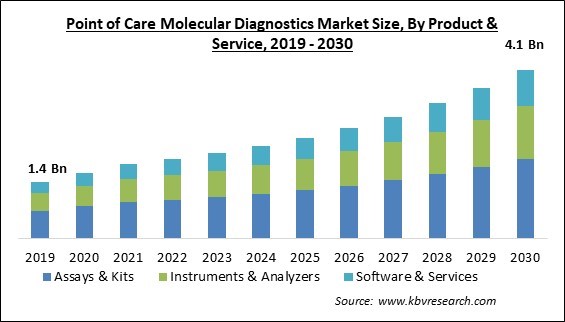
The Global Point of Care Molecular Diagnostics Market size is expected to reach $4.1 billion by 2030, rising at a market growth of 10.2% CAGR during the forecast period.
A new generation of POC devices that will change gastrointestinal diagnostic routes has been made possible by recent improvements in analytical methods. Hence, Gastrointestinal Disorders segment accounted for $113.1 million revenue in the market in 2022. About one-third of people in North European and North American populations still have Heliobacter pylori infection, compared to prevalence rates of more than 50% in South and East Europe, South America, and Asia. Immigrants from nations with high H. pylori prevalence continue to have a high prevalence of the stomach virus.
However, the younger generations' lower infection rates imply that the prevalence of H. pylori will continue to fall over the next several decades. The most significant risk factors for H. pylori infection are low socioeconomic situations throughout childhood. A hectic schedule is bringing on gastric ailments, an increase in the style of life, and poor eating habits. In 2018, there were reportedly 4.8 million new cases of cancer and 3.4 million deaths from it, according to the WHO, making up 26% of the burden of cancer incidence globally and 35% of all cancer-related fatalities. As a result, the prevalence of stomach illnesses would spur market expansion throughout the forecasted decade.
The major strategies followed by the market participants are Partnerships as the key developmental strategy to keep pace with the changing demands of end users. For instance, In March, 2023, QIAGEN N.V. formed a partnership with Servier to develop and validate a real-time in vitro PCR test that can be used to detect IDH1 gene mutations in AML patients in whole blood and bone marrow aspirates. Additionally, In February, 2021, Agilent Technologies formed a partnership with Mammoth Biosciences to offer a simple workflow to address the specific needs of high-throughput clinical testing laboratories.
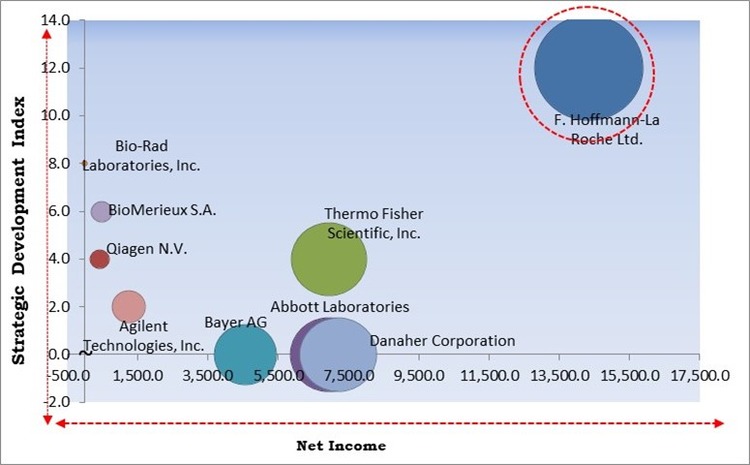
Based on the Analysis presented in the KBV Cardinal matrix; F. Hoffmann-La Roche Ltd. is the forerunner in the Market. In June, 2020, Roche formed a partnership with SpeeDx to enable Roche Diagnostics to offer clinicians critical and advanced tools for the detection of antibiotic resistance in patients with sexually transmitted infections. Companies such as Danaher Corporation, Thermo Fisher Scientific, Inc., Abbott Laboratories are some of the key innovators in the Market.
In practically every country, the percentage of older adults in the population is growing. WHO estimates that by 2030, 1 in 6 individuals worldwide will be 60 or older. By this point, there will be 1.4 billion people over 60, up from 1 billion in 2020. The number of individuals in the world who are 60 or older will double (to 2.1 billion) by 2050. Over the projection period, there will be an increase in demand for point-of-care molecular diagnostics due to the increased prevalence of respiratory illnesses and disorders among the aging population globally.
Due to the fast diagnostic outcomes necessary for precise and efficient treatment choices, point-of-care testing has become essential for patient-centric healthcare. Patients now have easy access to these diagnostics due to a change from centralized point-of-care testing to decentralized testing for chronic, infectious, and chronic conditions. Many players have created infectious disease tests for dispersed sites due to the demand for quick turnaround testing. These developments will favorably impact the growth of the worldwide market.
Inadequate reimbursements are a barrier to the point-of-care molecular diagnostics market's expansion. Among the major difficulties diagnostic firms encounter is receiving reimbursement for diagnostic tests from Medicare and commercial health insurers. In 2018, Medicare in the US changed how some in vitro diagnostic (IVD) tests, such as point-of-care molecular assays, were reimbursed. Diverse interest groups could increase a test's worth and serve as a persuasive lobby to generate reimbursement at the right amount. On the market for point-of-care molecular testing, these variables are projected to have a negative effect.
Based on product & service, the market is segmented into assays & kits, instruments & analyzers, and software & service. In 2022, the instruments & analyzers segment gained a sizable revenue share in the market. Plasma, urine, sputum, and swab samples are tested using molecular analyzers. Most molecular systems provide a broad range of test parameters, including those for TB, Chlamydia, HIV-1, HBV, and HCV. As a result, the equipment and analyzers market is expanding as more people get TBL, respiratory disorders, etc.
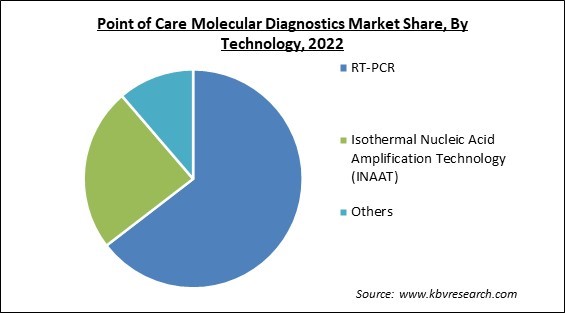
By technology, the market is divided into RT-PCR, Isothermal Nucleic Acid Amplification Technology (INAAT), and other technologies. The INAAT segment recorded a remarkable revenue share in the market in 2022. As a result of the rising prevalence of chronic diseases and an aging population, INAAT (Isothermal Nucleic Acid Amplification Technology) is projected to grow. An increase in the adoption of INAAT over PCR and a rise in demand for affordable and efficient diagnostic procedures are other key factors fueling the segment's expansion.
On the basis of application, the market is bifurcated into respiratory diseases, sexually transmitted diseases, hospital acquired infections, hepatitis, cancer, gastrointestinal disorders, and others. In 2022, the respiratory diseases segment dominated the market by generating the highest revenue share. The increasing frequency of infectious illnesses and the rising need for early diagnosis and identification of these diseases are the main factors fueling the expansion of this market segment.
By end-user, the market is categorized into physicians' offices, hospitals & ICUs, research institutes, and others. In 2022, the physicians' offices segment registered the largest revenue share in market. Due to its ability to provide quick findings within 30 minutes, POC molecular test & kits and systems are often used in these circumstances. This facilitates prompt diagnosis and enables doctors to determine or track a patient's status quickly.
| Report Attribute | Details |
|---|---|
| Market size value in 2022 | USD 1.9 Billion |
| Market size forecast in 2030 | USD 4.1 Billion |
| Base Year | 2022 |
| Historical Period | 2019 to 2021 |
| Forecast Period | 2023 to 2030 |
| Revenue Growth Rate | CAGR of 10.2% from 2023 to 2030 |
| Number of Pages | 314 |
| Number of Table | 493 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Product & Service, Technology, Application, End User, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Region wise, the market is analysed across North America, Europe, Asia Pacific, and LAMEA. In 2022, the North America region led the market with the maximum revenue share. One of the main factors influencing market expansion is the heavy load of malignancies and infectious illnesses on local healthcare systems. Other factors projected to boost the development of the point-of-care molecular diagnostics market in the area include the existence of a well-developed healthcare infrastructure and the rising acceptance of sophisticated technologies.
Free Valuable Insights: Global Point of Care Molecular Diagnostics Market size to reach USD 4.1 Billion by 2030
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Abbott Laboratories, Bayer AG, F. Hoffmann-La Roche Ltd., Qiagen N.V., Danaher Corporation, Bio-Rad Laboratories, Inc., BioMerieux S.A., Agilent Technologies, Inc., Thermo Fisher Scientific, Inc., and Nova Biomedical Corporation
By Product & Service
By End User
By Technology
By Application
By Geography
This Market size is expected to reach $4.1 billion by 2030.
An increasing number of older people are driving the Market in coming years, however, Poor reimbursement circumstances restraints the growth of the Market.
Abbott Laboratories, Bayer AG, F. Hoffmann-La Roche Ltd., Qiagen N.V., Danaher Corporation, Bio-Rad Laboratories, Inc., BioMerieux S.A., Agilent Technologies, Inc., Thermo Fisher Scientific, Inc., and Nova Biomedical Corporation
The Assays & Kits segment is generating the highest revenue share in the Global Point of Care Molecular Diagnostics Market by Product & Service in 2022, achieving a market value of $1.9 billion by 2030.
The RT-PCR segment is leading the Market by Technology in 2022, achieving a market value of $2.6 billion by 2030.
The North America market dominated the Market by Region in 2022 and would continue to be a dominant market till 2030; thereby, achieving a market value of $1.5 billion by 2030.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

