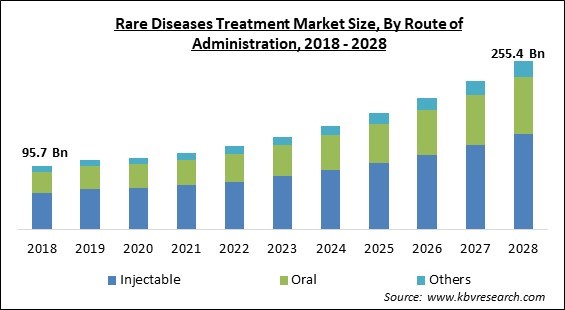
The Global Rare Diseases Treatment Market size is expected to reach $255.4 billion by 2028, rising at a market growth of 12.5% CAGR during the forecast period.
A rare disease is one that impacts a small proportion of the population. In some parts of the world, an orphan disease is a rare disease that lacks a large enough market to gain support & resources for uncovering treatments for it, unless the government grants economically advantageous conditions for developing and selling such treatments. Rare disease treatments are those that have been created or sold for this purpose.
A multidisciplinary approach based on innovative initiatives for the development of novel SMA drugs to address unmet needs in rare disease treatment would surge the demand for rare diseases treatment. In addition, basic research on patient access, clinical development challenges, and regulatory approval processes are major characteristics highlighted in policy proposals.
The condition frequently affects a patient's entire family, and continuous care is taxing. Even if only a small number of people are affected by a disease, it can put a massively disproportionate strain on healthcare & social services. The value chain can be extended to achieve forward integration. To achieve a high return on investment, operating efficiency is being increased. This can be accomplished by shifting the production of important medicines to orphan drugs.
The ongoing COVID-19 pandemic is a major public health concern that has presented numerous challenges to researchers, healthcare providers, and patients. Because of the risk of coronavirus exposure, R&D organizations and biopharma companies are not able to concentrate on the development of new treatments. To address such challenges, the FDA is proactively collaborating with manufacturers to provide advanced treatments to maintain health care continuity by focusing on ongoing clinical trials. As a result, during the pandemic period, drugs for rare disease treatments are all being developed in a very limited quantity and thus limiting the market growth for rare disease treatments in the upcoming years.
Because of the high prevalence of individuals suffering from rare diseases, special drugs will be required, which is accelerating the acceptance of rare disease treatments. These treatments are aimed at specific genes that have been transformed in order to cure the disease. Because of their simplicity and major non-invasive treatments, these treatments have grown in popularity. With this rise in popularity, rare disease treatment is expected to expand rapidly. Furthermore, these treatments are becoming more popular as healthcare infrastructure around the world improves.
The increasing R&D investments by popular vendors for orphan drug development of novel product offerings is one of the crucial driving factors prevailing in the market. Because public awareness and understanding of rare diseases have grown, a large number of key clinical-stage biopharmaceutical companies and established market players now have orphan drug pipeline candidates in various stages of clinical trials. This growing interest in rare disorder therapeutics is due to the fact that major pharmaceutical breakthroughs resulting in blockbuster drug developments are more likely in rare disorders than in traditional pharmaceutical portfolios.
Despite the increased focus on the development and marketing of rare disease therapeutics around the world, certain constraints are limiting the market growth. The high cost of researching and developing pipeline candidates for the treatment of rare diseases leads to an increase in drug prices. This huge cost of treatments has seriously limited the wide adoption of these product lines, particularly in developing countries. Such high treatment costs are also a source of concern in developed countries, where many drugs might not be adequately paid back, and hence, significantly increasing the patient's out-of-pocket costs.
By drug type, the rare disease treatment market is divided into biologics, biosimilar, and small molecule. The small molecule segment generated a substantial revenue share in the rare diseases treatment market in 2021. One of the factors influencing the demand for small molecule drugs is the small and medium-sized pharmaceutical companies' increasing demand for outsourcing. Also, the strict regulations on the development, manufacturing & quality control are further supporting the market growth in this segment.
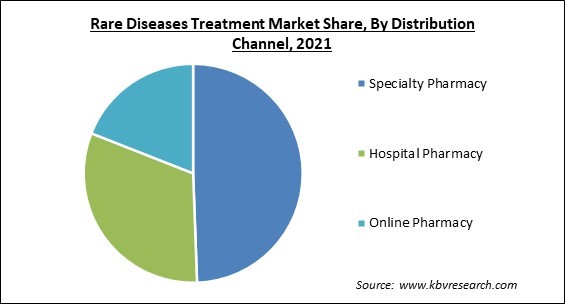
Based on distribution channel, the rare diseases treatment market is classified into hospital pharmacy, specialty pharmacy and online pharmacy. In 2021, the specialty segment witnessed the largest revenue share in the rare diseases treatment market. The growth is attributed to major players' strategic initiatives, like the acquisition of specialty pharmacies for the distribution of their drugs. During the forecast period, such mergers are anticipated to boost segment growth. Specialty drugs are extremely sophisticated drugs, usually based on biology, which structurally resemble substances found in the body.
On the basis of route of administration, the rare disease treatment market is fragmented into oral, injectable and others. The oral segment covered a significant revenue share in the rare disease treatment market in 2021. The market share is due to several benefits such as safety, compliance, ease of ingestion, and no risk of pain in the oral route of administration. As a result of growing awareness about the efficacy of such drugs, the oral route of administration has become more popular in the treatment of rare diseases.
Based on therapeutic area, the rare disease treatment market is segmented into cancer, neurological conditions, cardiovascular conditions, musculoskeletal conditions, hematologic disorders, infectious diseases, metabolic disorders, endocrine disorders and others. In 2021, the cancer segment dominated the rare disease treatment market by generating the highest revenue share. The high prevalence & recurrence rate of rare cancer evidence is the major factor responsible for this dominance. In addition, new treatments are continuously being updated for curing patients suffering from rare cancers.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 115.4 Billion |
| Market size forecast in 2028 | USD 255.4 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 12.5% from 2022 to 2028 |
| Number of Pages | 308 |
| Number of Tables | 503 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Drug Type, Distribution Channel, Route of Administration, Therapeutic Area, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Region wise, the rare disease treatment market is analyzed across the North America, Europe, Asia Pacific and LAMEA. In 2021, the North America region dominated the rare diseases treatment market with the maximum revenue share. This is because of the high prevalence of diseases, favorable healthcare infrastructure, and new product approvals for treatment. Access to products may enhance patient compliance, thereby expanding the customer base in the nations such as Canada, and the United States are propelling the growth of rare disease treatment in the North America region.
Free Valuable Insights: Global Rare Diseases Treatment Market size to reach USD 255.4 Billion by 2028
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include AbbVie, Inc., F. Hoffmann-La Roche Ltd., Bayer AG, Novartis AG, Merck Group, Bristol Myers Squibb Company, Pfizer, Inc., AstraZeneca PLC, Takeda Pharmaceutical Company Limited and PTC Therapeutics, Inc.
By Drug Type
By Distribution Channel
By Route of Administration
By Therapeutic Area
By Geography
The Rare Diseases Treatment Market size is projected to reach USD 255.4 billion by 2028.
The increasing presence of rare diseases are driving the market in coming years, however, High costs involved in R&D of the rare diseases treatment restraints the growth of the market.
AbbVie, Inc., F. Hoffmann-La Roche Ltd., Bayer AG, Novartis AG, Merck Group, Bristol Myers Squibb Company, Pfizer, Inc., AstraZeneca PLC, Takeda Pharmaceutical Company Limited and PTC Therapeutics, Inc.
The Injectable market has high growth rate in Global Rare Diseases Treatment Market by Route of Administration in 2021, thereby, achieving a market value of $144.25 billion by 2028.
The Biologics market is leading the Global Rare Diseases Treatment Market by Drug Type in 2021, thereby, achieving a market value of $141 billion by 2028.
The North America market is leading the Global Rare Diseases Treatment Market by Region in 2021, thereby, achieving a market value of $90.7 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

