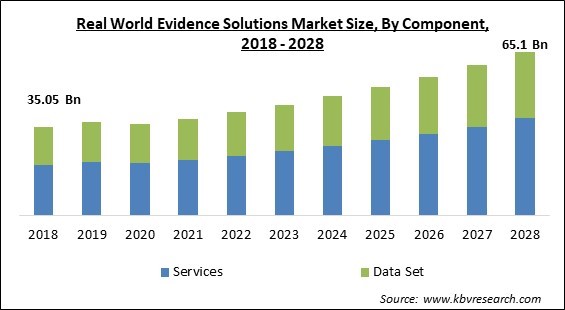
The Global Real World Evidence Solutions Market size is expected to reach $65.1 billion by 2028, rising at a market growth of 8.0% CAGR during the forecast period.
In medicine, clinical evidence about the use and possible advantages or dangers of a medical product that is produced from a study of real-world data (RWD) is known as real-world evidence (RWE). Different analyses or study designs, such as randomized trials, including pragmatic trials, large simple trials, and prospective or retrospective observational studies, among others, might produce RWE. When clinical trials cannot adequately represent the whole patient population for a given disease, real-world evidence is required.
Patients who did not take part in any clinical trials, have comorbid conditions, reside in a remote location, or are older than the recommended age range may not react to the medication in consideration as expected. RWE offers solutions to these issues as well as the ability to examine the effects of medications over a longer time frame. Pharmaceutical businesses, as well as health insurance payers, research RWE in order to comprehend patient pathways, provide appropriate care for the right people, and reduce their financial risk by funding patient-effective drugs.
The World Ageing Population report estimates that 703 million individuals worldwide were 65 years of age or older in 2019, and that figure is projected to rise to 1.5 billion by 2050. According to estimates, cancer, obesity, cardiovascular diseases, and diabetes, all of which are on the rise, are responsible for over half of all fatalities from chronic diseases. Pharmaceutical corporations spend billions on research & development with a minimal chance of success before bringing a new medication molecule to market.
Pharma research facilities need a strong understanding of treatment choices that are effective across a large population as opposed to small patient groups in clinical trials if they are to shorten drug development cycles. Thus, it is discovered that the usage of real-world evidence (RWE) findings is growing. This is because pharmaceuticals and biotech companies are becoming more aware of the potential value of RWE in life-cycle management and drug development, as well as how it can lower the cost of clinical trials and boost innovation efficiency.
The infectious coronavirus could economically impact most industries, including the pharmaceutical sector. Many medical professionals and pharmaceutical companies have used their resources to stop the COVID-19 pandemic's spread and create efficient drugs and vaccinations. Thus, a tendency toward patient-centered virtual care and digitalization is being observed in the industry. This highlights the requirement for practical, workable solutions. Two prominent healthcare data science firms, Aetion and HealthVerity, were discovered partnering in 2020, strengthening their already established partnership to extract practical medical evidence from EHRs (electronic health records) and counsel drug producers and authorities on COVID-19 treatments.
Most biopharmaceutical, pharmaceutical, and medical device businesses continue to make significant investments in creating new medications and equipment. R&D is very important in the pharmaceutical business in particular. Pharmaceutical and biopharmaceutical businesses are choosing fully functional or integrated outsourcing services from the early development phase to the late-stage development phase to meet the requirements for drug development and discovery due to their increasing R&D spending. The pharmaceutical sector of the economy spends the highest proportion of its profits on research and development.
Healthcare stakeholders are looking for new solutions to deal with the unaffordable financial burden and relatively low return on investment, transforming the healthcare ecosystem and raising concerns about healthcare prices. Businesses require robust evidence lifecycle management skills to demonstrate value. In turn, this has increased the possibility of using an end-to-end strategy to leverage a life sciences organization's data, proof, and knowledge resources. Thus, aiding in the dismantling of traditional silos and enabling insight-driven decision-making from the product R&D stage to its commercialization. Hence, the rising emphasis on using comprehensive RWE data is propelling the market's growth.
Non-randomized observational studies have historically not been seen as appropriate for evaluating novel medical interventions or treatments due to methodological and research design concerns, such as the possibility of confounding and bias. RWE has spread quickly across applications, yet some stakeholders still hesitate to rely on them. For example, although payers have begun employing RWE, they opt to use internal observational data from randomized clinical trials (RCTs) to influence drug coverage choices. Even a study that is robust, well-planned, and non-randomized would be seen as lower-quality evidence in comparison to an RCT that is poorly conceived. These factors are preventing the Real-World Evidence Solutions Market from expanding.
Based on Component, the Real World Evidence Solutions Market is segmented into Services and Data Sets. The segment's growth is linked to the widespread use of real-world evidence services by healthcare organizations and businesses involved in biotechnology and pharmaceuticals. Presently, Syneos Health, IQVIA, and ICON are providing late-phase services like research planning, protocol development, clinical study management & reporting, as well as endwise real-world evidence (RWE) solutions. Real-world evidence (RWE) solutions vendors may have more opportunities to increase their investments during the medication development cycle due to the growing demand for comprehensive evidence services all throughout the product lifetime.
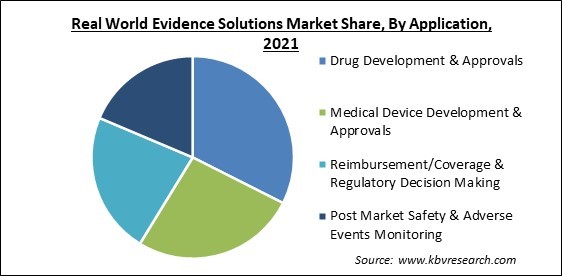
Based on Application, the Real World Evidence Solutions Market is segmented into Drug Development & Approvals, Medical Device Development & Approvals, Reimbursement/Coverage & Regulatory Decision Making, and Post Market Safety & Adverse Events Monitoring. The reimbursement/coverage and regulatory decision making segment recorded a substantial revenue share in the real world evidence solutions market in 2021. The increasing emphasis on clinical value evidence for choices to offer coverage and the rising cost of therapy is responsible for expanding the reimbursement/coverage and regulatory decision-making segment. The post-market safety and adverse event monitoring area are also projected to expand significantly.
Based on End-user, the Real World Evidence Solutions Market is segmented into Healthcare Companies, Healthcare Payers, Healthcare Providers, and Others. The healthcare companies segment witnessed the maximum revenue share in the real world evidence solutions market in 2021. The surge is linked to the growing significance of RWE studies in determining how well drugs operate in actual situations and mitigating drug recalls. RWE can assist and advance several processes, including determining which patient group will benefit from an intervention the most, assisting with regulatory approvals and completing data from randomized controlled trials, and assisting with reimbursement efforts and expanding usage indications.
Based on Therapeutic Area, the Real World Evidence Solutions Market is segmented into Oncology, Cardiology, Neurology, Diabetes, Respiratory, and Others. The diabetes segment witnessed a significant revenue share in the real world evidence solutions market in 2021. Further understanding of the efficacy and safety of medications for diabetes and certain other diseases for which diabetes can be an underlying causative factor is necessary. This insight, in clinical settings, can be gained by combining data from randomized controlled trials with evidence from real-world settings.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 38.5 Billion |
| Market size forecast in 2028 | USD 65.1 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 8% from 2022 to 2028 |
| Number of Pages | 293 |
| Number of Tables | 479 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Component, Therapeutic Area, Application, End-user, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Based on Region, the Real World Evidence Solutions Market is segmented into North America, Europe, Asia Pacific, and LAMEA. The Asia Pacific segment procured a considerable growth rate in the real world evidence solutions market in 2021. Increased government initiatives to implement RWE studies and numerous contract research organizations (CROs) and contract research manufacturing organizations in nations like China and India are responsible for this region's rise. Additionally predicted to drive market growth is rising consumer desire for better healthcare services.
Free Valuable Insights: Global Real World Evidence Solutions Market size to reach USD 65.1 Billion by 2028
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include IBM Corporation, Oracle Corporation, Medpace Holdings, Inc., IQVIA Holdings, Inc., ICON plc, PerkinElmer, Inc., Parexel International Corporation (Phoenix Parentco, Inc.), PPD, Inc. (Thermo Fisher Scientific, Inc.), UnitedHealth Group, Inc. (Optum, Inc.), and Syneos Health, Inc.
By Component
By Therapeutic Area
By End-user
By Application
By Geography
The global Real World Evidence Solutions Market size is expected to reach $65.1 billion by 2028.
Rising investment in R&D to create new goods and medical devices along with lowering the price of medication development are driving the market in coming years, however, Medical professionals' and researchers' reluctance to depend on real-world studies restraints the growth of the market.
IBM Corporation, Oracle Corporation, Medpace Holdings, Inc., IQVIA Holdings, Inc., ICON plc, PerkinElmer, Inc., Parexel International Corporation (Phoenix Parentco, Inc.), PPD, Inc. (Thermo Fisher Scientific, Inc.), UnitedHealth Group, Inc. (Optum, Inc.), and Syneos Health, Inc.
The Oncology segment acquired maximum revenue share in the Global Real World Evidence Solutions Market by Therapeutic Area in 2021 thereby, achieving a market value of $18.4 billion by 2028.
The Drug Development & Approvals segment is leading the Global Real World Evidence Solutions Market by Application in 2021 thereby, achieving a market value of $19.2 billion by 2028.
The North America market dominated the Global Real World Evidence Solutions Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $26.9 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

