“Global Regulatory Information Management System Market to reach a market value of USD 4.7 Billion by 2031 growing at a CAGR of 10.5%”
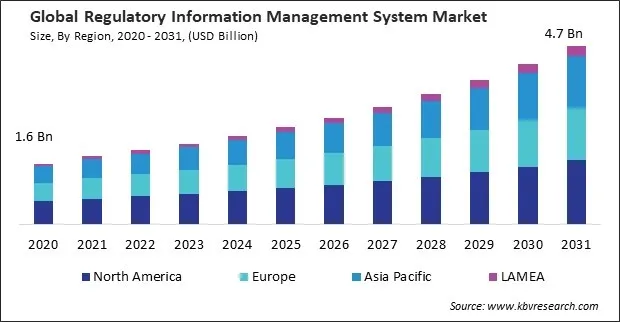
The Global Regulatory Information Management System Market size is expected to reach $4.7 billion by 2031, rising at a market growth of 10.5% CAGR during the forecast period.
The Asia Pacific region is home to rapidly growing pharmaceutical and healthcare industries. Thus, the Asia Pacific region acquired $579.5 million revenue in 2023. As the pharmaceutical sector expands, the complexity of regulatory compliance requirements also increases, driving the demand for robust regulatory information management systems to ensure compliance with regulatory standards, streamline regulatory processes, and accelerate product approvals in China.
Quality management is paramount in the pharmaceutical and healthcare industries to ensure that products meet safety and efficacy standards. RIMS solutions integrate with Quality Management Systems (QMS) to support quality management processes such as document control, deviation management, corrective and preventive actions (CAPA), and audit management. Therefore, the market is expanding significantly due to the rising focus on patient safety and product quality.
Additionally, RIMS solutions facilitate collaboration among cross-functional teams involved in regulatory processes, including regulatory affairs, quality assurance, clinical research, and product development. RIMS enables teams to collaborate effectively, streamline communication, and coordinate efforts to achieve regulatory objectives by providing a centralized platform for sharing regulatory information, documents, and submissions. Thus, because of the enhanced collaboration and interoperability, the market is anticipated to increase significantly.
However, SMEs often operate with tighter budgets compared to larger corporations. The high upfront costs associated with implementing RIMS solutions may deter SMEs from investing in these systems, limiting their ability to enhance regulatory compliance, streamline processes, and compete effectively in regulated markets. Thus, high implementation costs can slow down the growth of the market.
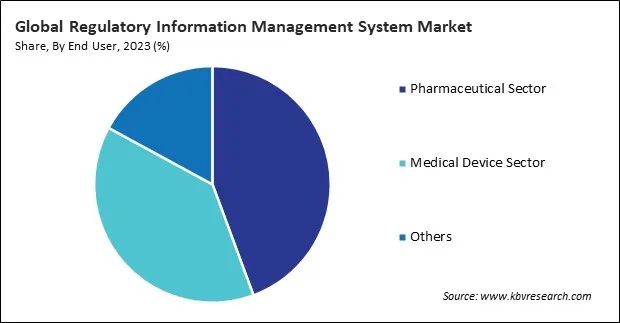
By end user, the market is categorized into pharmaceutical sector, medical device sector, and others. In 2023, the pharmaceutical sector segment held a 44.3% revenue share in the market. Pharmaceutical companies must submit various regulatory documents to health authorities for drug approval and marketing authorization.
Free Valuable Insights: Global Regulatory Information Management System Market size to reach USD 4.7 Billion by 2031
Region-wise, the market is analysed across North America, Europe, Asia Pacific, and LAMEA. In 2023, the North America region led the market by generating the highest 40% revenue share. Regulatory agencies in North America prioritize patient safety and product quality in approving and overseeing pharmaceuticals, medical devices, and healthcare products.
| Report Attribute | Details |
|---|---|
| Market size value in 2023 | USD 2.1 Billion |
| Market size forecast in 2031 | USD 4.7 Billion |
| Base Year | 2023 |
| Historical Period | 2020 to 2022 |
| Forecast Period | 2024 to 2031 |
| Revenue Growth Rate | CAGR of 10.5% from 2024 to 2031 |
| Number of Pages | 147 |
| Number of Tables | 170 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Porter’s 5 Forces Analysis, Company Profiling, Companies Strategic Developments, SWOT Analysis, Winning Imperatives |
| Segments covered | End User, Region |
| Country scope |
|
| Companies Included | Veeva Systems, Inc., Korber AG (Optel Group), ArisGlobal LLC (Nordic Capital Limited), Calyx, Ennov SAS, MasterControl, Inc., LORENZ Life Sciences Group, AmpleLogic, Cencora, Inc. (PharmaLex Holding GmbH), Ithos Global Inc. (Cordance Group) |
By End User
By Geography
This Market size is expected to reach $4.7 billion by 2031.
Rising focus on patient safety and product quality are driving the Market in coming years, however, Data security and privacy concerns restraints the growth of the Market.
Veeva Systems, Inc., Korber AG (Optel Group), ArisGlobal LLC (Nordic Capital Limited), Calyx, Ennov SAS, MasterControl, Inc., LORENZ Life Sciences Group, AmpleLogic, Cencora, Inc. (PharmaLex Holding GmbH), Ithos Global Inc. (Cordance Group)
The expected CAGR of this Market is 10.5% from 2024 to 2031.
The North America region dominated the Market by Region in 2023; thereby, achieving a market value of $1.7 billion by 2031.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

 Drivers
Drivers
 Restraints
Restraints
 Opportunities
Opportunities
 Challenges
Challenges
