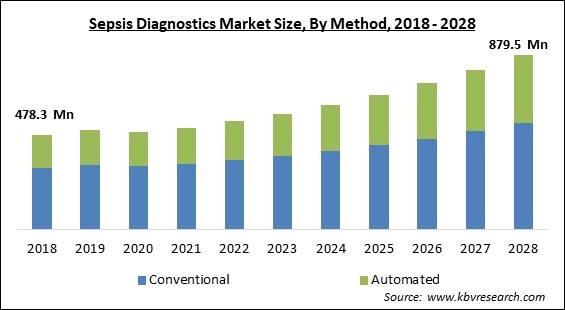
The Global Sepsis Diagnostics Market size is expected to reach $879.5 million by 2028, rising at a market growth of 8.3% CAGR during the forecast period.
Sepsis is a medical disorder that emerges as a result of the body's response to infections caused by bacteria, viruses, or fungus. The immune system produces various substances into the bloodstream in this situation, causing hyper inflammation and the production of blood clots. Multiple organ failures, rapid breathing, neurological dysfunction, low blood pressure, and a higher heart rate are all possible complications of sepsis. Molecular diagnosis, biomarkers, flow cytometry, immunoassays, microfluidics, and other processes are used to diagnose sepsis. Hospitals, pathology and reference laboratories, university academic researchers, and other institutions are all using these diagnostic tools.
In 2016, the Third International Sepsis Consensus Task Force determined the current criteria for clinical sepsis diagnosis, known as Sequential Organ Failure Assessment (SOFA). This scoring system detects cumulative organ dysfunction as a result of an infection-induced dysregulation of the host's immune system. Cardiovascular function, respiration, liver function, coagulation, central nervous system function, and renal function are all regarded in the SOFA score.
A point is given for each malfunctioning organ system, with a score of 2 points or higher indicating a definite sepsis diagnosis. A high SOFA score is linked to a higher than 10% hospital mortality risk. Quick SOFA (qSOFA) is a rapid alternative to SOFA that uses only a subset of the SOFA scoring criteria, such as abnormal mentation, systolic blood pressure under 100 mm Hg, and a breathing rate of 22 or more per minute. qSOFA does not necessitate laboratory testing and can be performed as many times as needed.
SOFA replaces the Systemic Inflammatory Response Syndrome (SIRS) criteria, which was created in 2001. SIRS scoring requires two or more of the following: a temperature of greater than 38°C or less than 36°C, a heart rate of greater than 90 beats per minute, a respiratory rate of greater than 20 beats per minute, or a white blood cell count of greater than 12,000/L or less than 4,000/L. When compared to SIRS and qSOFA, SOFA has higher predictive accuracy.
The COVID-19 pandemic had a marginally beneficial impact on sepsis diagnostics sales. The widespread of COVID-19 and the emergence of sepsis cases among COVID-19 patients are projected to boost the demand for quick diagnosis, speeding up the use of tools, reagents, and test kits for sepsis detection. COVID-19 infection is more likely to affect surgeons and patients undergoing a variety of procedures in hospitals. The bulk of sepsis diagnostics was halted, leading to a decrease in device-based treatments. People postponed health exams as a result of the pandemic's lockdowns, lowering the number of tests conducted and laboratory sales.
HAIs also characterized as nosocomial infections, a prominent cause of morbidity and mortality in hospitals across the world. The most common hospital-acquired illnesses include urinary tract infections, pneumonia, and sepsis (HAIs). HAIs can induce sepsis in immunocompromised people, the elderly, and people with medical conditions. According to the Centres for Disease Control and Prevention (CDC), sepsis affects at least 1.7 million adults in the United States each year, with about 270,000 deaths. An illness obtained at a medical facility, such as an inpatient setting or a skilled nursing care facility, is characterized as a healthcare-acquired infection (HAI). An HAI might come from anywhere, even a doctor's office or clinic. HAIs are known as nosocomial infections in the medical world. Infections acquired outside of a healthcare centre are known as community-acquired infections.
Due to their ease of use and effectiveness in diagnostic procedures, molecular diagnostics has attracted a number of multinational companies and institutes. The gold standard for molecular diagnostic methods has been blood culture analysis for quick and precise infection profiling. Several firms are investing in point-of-care molecular diagnostics to improve patient management and early detection of bacterial and viral infections. For example, Immunexpress debuted a quick SeptiCyte, a one-hour molecular diagnostic test for sepsis, on Biocartis' Idylla platform in October 2020. This is one of the first completely integrated, immune response-based diagnostics to help clinicians diagnose sepsis.
Companies are focusing on creating automated diagnostic instruments for identifying sepsis based on modern technologies such as molecular diagnostics. However, government hospitals and scientific research laboratories cannot acquire such systems due to budget constraints. Furthermore, it is becoming a key impediment to the expansion of the sepsis diagnostics market. In addition, the lowest intensity sepsis cohort population had the major burden of recurrence and total expenses. Sepsis cases that were not discovered until hospitalization, along with those with increasing severity, had a greater economic cost and death rate on a case-by-case basis.
Based on Method, the market is segmented into Conventional and Automated. The automated segment witnessed a substantial revenue share in the Sepsis Diagnostics Market in 2021. The use of billing codes and the installation of predictable evaluation are based on agreement definitions that have been used in efforts to automate sepsis identification. Although billing codes are simple to extract and have a strong positive predictive value (PPV) for sepsis, they typically do not document the onset of sepsis and have low sensitivity when compared to clinician assessment. Changing medical practicing habits and reimbursement policies also have an impact on them. Consensus-based definitions have improved over time and across institutions, but they still rely on doctors to validate if organ dysfunction is attributable to sepsis, resulting in low PPV when automated.
Based on Test Type, the market is segmented into Laboratory Tests and Point-of-Care Tests. The laboratory tests procured the highest revenue share in the Sepsis Diagnostics Market in 2021. This is mostly attributable to the fact that laboratory testing is more accurate than POC testing when it comes to identifying sepsis. Laboratory testing allows for a comprehensive examination of the pathogen and its medication resistance profile. In addition, routine lactate testing, which is a vague but valuable indication of sepsis, is done efficiently in laboratory settings. The public's and physicians' dependence on these labs has grown dramatically with the introduction of technologically advanced products.
Based on Pathogen, the market is segmented into Bacterial Sepsis, Fungal Sepsis, Viral Sepsis, and Others. The fungal sepsis recorded a substantial revenue share in the Sepsis Diagnostics Market in 2021. Fungi are responsible for about 15% of all infections, and invasive fungal infections are becoming a more common cause of sepsis, especially in critically ill patients. This disease are life-threatening illness that can occur anywhere in the body as a result of an infection. Severe instances of sepsis are frequently caused by infections that spread throughout the body and through the bloodstream.
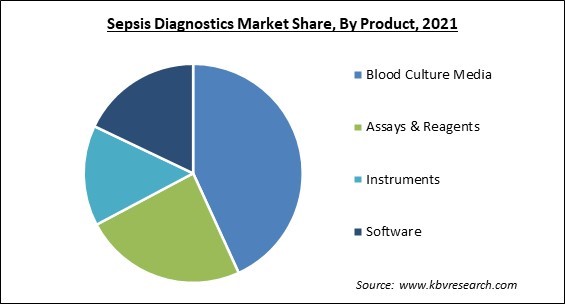
Based on Product, the market is segmented into Blood Culture Media, Assays & Reagents, Instruments, and Software. The software segment registered a substantial revenue share in the Sepsis Diagnostics Market in 2021. This rapid expansion of this segment can be ascribed to a rise in healthcare professionals' implementation of modern software platforms for better sepsis detection and management. For example, Guthrie, anintegrated health care system, used Wolters Kluwer's POC Advisor to diagnose and treat sepsis at four local hospitals in September 2019.
Based on End-User, the market is segmented into Hospitals & specialty clinics, Pathology & Reference Laboratories, and Research Laboratories & Academic Institutes. The hospitals and specialty clinics segment procured the highest revenue share in the Sepsis Diagnostics Market in 2021. The rising number of patients diagnosed with sepsis, and in-house hospital and clinics are undertaking a large number of blood culture tests to identify blood stream infections (BSIs caused by bacteria, fungi/yeast, or viruses) contribute to the increased growth of this segment.
Based on Technology, the market is segmented into Blood Culture, Immunoassays, Molecular Diagnostics, Flow Cytometry, Microfluidics, and Biomarkers. The blood culture segment garnered the largest revenue share in the Sepsis Diagnostics Market in 2021. One of the most significant investigations in the treatment of sepsis is blood culture. It enables the identification of the sepsis-causing bacterium, the selection of a relevant empirical and specific antibiotic, and the identification of the infection's focal location. All sepsis patients should have a blood culture taken. Sepsis is traditionally described as the presence of infection-induced systemic inflammatory response syndrome (SIRS). Moreover, the affordable pricing of microbiological techniques and the widespread usage of blood culture procedures for sepsis diagnosis also account for this proportion.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 511.3 Million |
| Market size forecast in 2028 | USD 879.5 Million |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 8.3% from 2022 to 2028 |
| Number of Pages | 378 |
| Number of Tables | 655 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Method, Test Type, Pathogen, Product, End-User, Technology, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. The North America segment acquired the highest revenue share in the Sepsis Diagnostics Market in 2021. The medical device market in North America is well-established. The presence of a well-developed healthcare system, widespread implementation of innovative sepsis diagnosing technologies between health personnel, a reducing number of surgical procedures, growing prevalence of healthcare-associated infections (HAIs), and adoption of advanced technologies of sepsis diagnostics are all key factors that drive the sepsis diagnostics market in North America.
Free Valuable Insights: Global Sepsis Diagnostics MarketMarket size to reach USD 879.5 Million by 2028
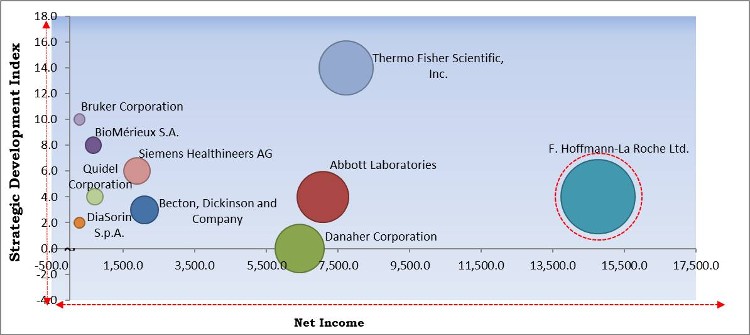
The major strategies followed by the market participants are Acquisitions. Based on the Analysis presented in the Cardinal matrix; F. Hoffmann-La Roche Ltd. is the major forerunner in the Sepsis Diagnostics Market. Companies such as Thermo Fisher Scientific, Inc., Bruker Corporation and BioMérieux S.A. are some of the key innovators in the Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include BioMérieux S.A., F. Hoffmann-La Roche Ltd., Becton, Dickinson and Company, Abbott Laboratories, Thermo Fisher Scientific, Inc., DiaSorin S.p.A., Bruker Corporation, Siemens Healthineers AG, Danaher Corporation, and Quidel Corporation.
By Method
By Test Type
By Pathogen
By Product
By End-User
By Technology
By Geography
The global sepsis diagnostics market size is expected to reach $879.5 million by 2028.
A rise in the number of hospital-acquired illnesses are increasing are driving the market in coming years, however, high expense of automated diagnostic instruments growth of the market.
BioMérieux S.A., F. Hoffmann-La Roche Ltd., Becton, Dickinson and Company, Abbott Laboratories, Thermo Fisher Scientific, Inc., DiaSorin S.p.A., Bruker Corporation, Siemens Healthineers AG, Danaher Corporation, and Quidel Corporation.
The Conventional segment acquired maximum revenue share in the Global Sepsis Diagnostics Market by Method in 2021, thereby, achieving a market value of $538.7 million by 2028.
The Bacterial Sepsis segment is leading the Global Sepsis Diagnostics Market by Pathogen in 2021, thereby, achieving a market value of $324.4 million by 2028.
The North America is the fastest growing region in the Global Sepsis Diagnostics Market by Region in 2021, and would continue to be a dominant market till 2018.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

