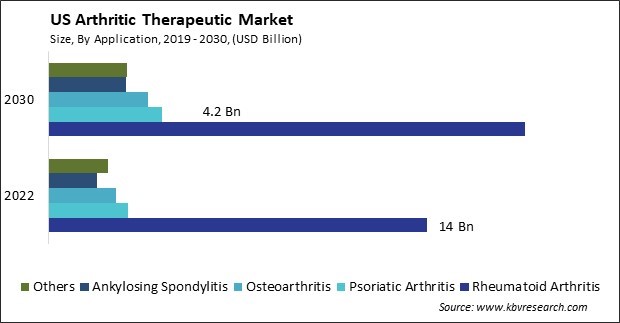
The US Arthritic Therapeutic Market size is expected to reach $31.3 Billion by 2030, rising at a market growth of 3.8% CAGR during the forecast period.
The arthritic therapeutic market in the United States is a complex and dynamic landscape shaped by various factors including demographics, advancements in medical research, regulatory policies, and the evolving needs of patients. The prevalence of arthritis in the United States is considerable and is expected to rise with the aging population. According to the Centers for Disease Control and Prevention (CDC), an estimated 54.4 million adults had doctor-diagnosed arthritis in 2019, with projections suggesting this number will escalate to 78.4 million by 2040. This demographic shift underscores the growing demand for effective therapeutic interventions to manage symptoms and improve the quality of life for affected individuals.
Furthermore, the pharmaceutical industry remains at the forefront of innovation in arthritic therapeutics, with ongoing research and development efforts focused on novel drug targets, formulations, and delivery mechanisms. According to the Pharmaceutical Research and Manufacturers Association (PhRMA), in 2019, foreign direct investment in pharmaceuticals totaled $511.3 billion. Biologic therapies have revolutionized the treatment landscape in the U.S. by targeting specific components of the immune system involved in arthritis pathogenesis.
However, the COVID-19 pandemic has significantly impacted the arthritic therapeutic market in the United States. Patients with arthritis, particularly those on immunosuppressive medications like biologics or DMARDs, faced heightened concerns about their susceptibility to the virus and its potential complications. As a result, healthcare providers and patients had to navigate the delicate balance between managing arthritis symptoms and minimizing the risk of COVID-19 exposure.
The pandemic also disrupted healthcare delivery systems, leading to delays in routine care, elective procedures, and clinical trials related to arthritic therapeutics. Telemedicine emerged as a valuable tool for remote consultations and monitoring, offering a way to maintain continuity of care while adhering to social distancing guidelines. However, challenges related to digital literacy, access to technology, and reimbursement policies posed barriers to widespread adoption.
In recent years, there has been a significant increase in awareness surrounding arthritis, driving growth in the arthritic therapeutic market. One of the primary drivers of increased awareness is the proactive efforts of healthcare professionals, advocacy groups, and governmental organizations to educate the public about arthritis. Through campaigns, workshops, and online resources, they aim to raise awareness about the symptoms, risk factors, and available treatment options for arthritis in the U.S.
Furthermore, the aging population in the U.S. has contributed to the rising prevalence of arthritis, further fueling awareness and demand for effective treatments. According to the United States Census Bureau, the aging demographic trend in the United States has been remarkable, with the older population swelling from 4.9 million in 1920 to a staggering 55.8 million individuals, constituting 16.8% of the population in 2020. As people live longer, they are more likely to develop age-related conditions like osteoarthritis, the most common form of arthritis. This demographic shift has prompted healthcare providers to prioritize arthritis management strategies and invest in research and development efforts to address the evolving needs of aging populations.
Moreover, biologic therapies, for instance, target specific molecules involved in the inflammatory process, offering promising outcomes for patients with severe forms of arthritis. Additionally, the emergence of regenerative medicine techniques, such as stem cell therapy and platelet-rich plasma injections, holds potential for repairing damaged joint tissues and alleviating symptoms in arthritis patients. Therefore, increased awareness initiatives, demographic shifts, and advancements in therapeutic approaches, including biologics and regenerative medicine, are driving growth in the arthritic therapeutic market in the U.S.
The adoption of disease-modifying anti-rheumatic drugs (DMARDs) in the arthritic therapeutic market in the United States has seen significant growth in recent years. One of the primary drivers of the increased adoption of DMARDs is the growing recognition of their efficacy in managing anti-rheumatic (RA). DMARDs target the disease's underlying mechanisms, such as inflammation and immune system dysfunction, rather than just alleviating symptoms. This approach relieves pain and swelling, helps slow disease progression, and prevents joint damage, ultimately improving patient outcomes.
Another factor contributing to the rising adoption of DMARDs is the expansion of patient treatment options. Traditionally, conventional synthetic DMARDs like methotrexate were the mainstay of anti-rheumatic treatment. Moreover, the emphasis on early and aggressive treatment strategies in RA management guidelines has underscored the importance of initiating DMARD therapy promptly after diagnosis. Early intervention with DMARDs has improved outcomes, including reducing joint damage, preserving physical function, and enhancing the quality of life for patients with RA.
Additionally, healthcare providers' growing awareness of the impact of RA on patients' overall health and well-being has led to a greater emphasis on comprehensive, personalized treatment plans that include DMARDs as a central component. Hence, the increasing recognition of DMARD efficacy in managing rheumatoid arthritis, expanded treatment options, and emphasis on early intervention have driven significant growth in their adoption within the United States arthritic therapeutic market.
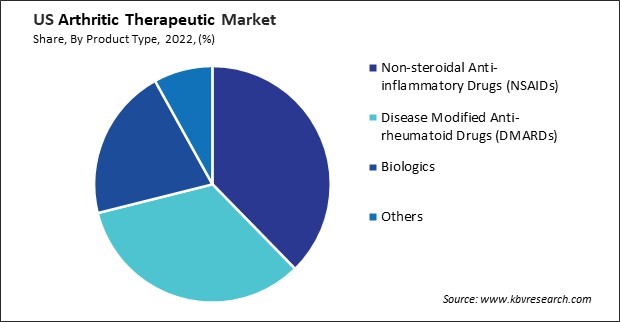
The arthritic therapeutic market in the United States is a significant sector within the broader pharmaceutical and healthcare industry. One prominent player in the arthritic therapeutic market is Pfizer Inc. Pfizer develops and manufactures a range of medications used in the treatment of arthritis, including nonsteroidal anti-inflammatory drugs (NSAIDs) such as Celebrex (celecoxib) and Xeljanz (tofacitinib). These medications reduce inflammation and pain associated with various forms of arthritis, including rheumatoid arthritis and osteoarthritis. Pfizer's extensive research and development efforts continue to drive innovation in arthritic therapeutics.
Biogen Inc. is a biotechnology company that has made notable contributions to the arthritic therapeutic market with its Ocrevus (ocrelizumab) drug. While primarily indicated for multiple sclerosis, Ocrevus has shown promise in treating certain forms of arthritis by targeting B cells involved in the inflammatory process. Biogen's ongoing research into the immunological mechanisms underlying arthritis holds the potential for developing novel treatments in the future.
Another key player in the U.S. arthritic therapeutic market is AbbVie Inc. AbbVie is known for its biologic medications, including Humira (adalimumab), one of the most widely prescribed drugs for rheumatoid arthritis. Humira belongs to a class of drugs called tumor necrosis factor (TNF) inhibitors, which help reduce inflammation by targeting specific proteins in the immune system. AbbVie's strong focus on biologics and ongoing investment in research contribute to its leadership in the arthritic therapeutic space.
Amgen Inc. is another key player in the arthritic therapeutic market, with medications such as Enbrel (etanercept) and Prolia (denosumab) in its portfolio. Enbrel, like Humira and Remicade, is a TNF inhibitor used to manage rheumatoid arthritis and other autoimmune conditions. Prolia, on the other hand, is a monoclonal antibody indicated for the treatment of osteoporosis, a common comorbidity of arthritis. Amgen's diverse product offerings address different aspects of arthritis management, catering to the needs of a broad patient population.
In addition to these major pharmaceutical companies, there are numerous smaller biotech firms and generic drug manufacturers that contribute to the arthritic therapeutic market in the U.S. through the development and production of medications, including pain relievers, disease-modifying anti-rheumatic drugs (DMARDs) and biologics biosimilars. The competitive landscape of the arthritic therapeutic market is dynamic, with companies continually striving to innovate and improve treatment options for individuals affected by arthritis. Hence, the arthritic therapeutic market in the U.S. is characterized by a diverse array of pharmaceutical companies and biotech firms dedicated to addressing the needs of patients with arthritis.
By Application
By Product Type
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

