The United States (US) Mycoplasma Testing Market size is expected to reach $541.8 Million by 2030, rising at a market growth of 10.1% CAGR during the forecast period.
The mycoplasma testing market in the United States has witnessed significant growth in recent years, driven by the increasing awareness and importance of mycoplasma contamination in cell culture processes, biopharmaceutical production, and research laboratories. One of the key factors contributing to the growth of the mycoplasma testing market in the U.S. is the rising adoption of cell-based research and biopharmaceutical manufacturing.
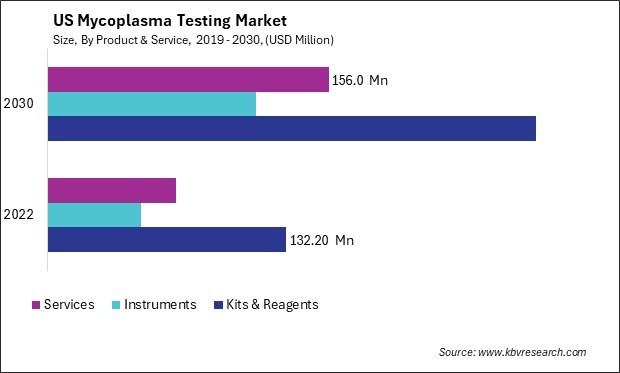
With the ongoing advancements in biotechnology and pharmaceutical research, the mycoplasma testing market in the U.S. is expected to continue its growth trajectory. The need for accurate and efficient testing solutions to ensure the integrity of cell cultures and biopharmaceutical products will remain a driving force, making the U.S. a significant hub for mycoplasma testing advancements and solutions. The U.S. mycoplasma testing market is characterized by several key players offering various testing kits, reagents, and services. These companies are focusing on innovations to enhance the sensitivity and specificity of their testing products. Furthermore, collaborations and partnerships between industry players and research institutions contribute to developing advanced testing methodologies.
As the biopharmaceutical industry in the U.S. continues to witness growth and innovation, the need for mycoplasma testing is expected to persist and expand. Emerging biotechnologies, such as cell and gene therapies, present new challenges and opportunities for mycoplasma testing, as these advanced therapies involve complex cell cultures. Consequently, the mycoplasma testing market in the U.S. is likely to witness sustained growth as these cutting-edge therapies become more prevalent in the healthcare landscape.
The outbreak of the COVID-19 pandemic has also left a significant impact on the mycoplasma testing market in the United States. The disruption caused by the pandemic has led to delays in research and development activities across various industries. With laboratories prioritizing COVID-19-related research, other essential areas, including mycoplasma testing, experienced a slowdown. However, the increased emphasis on biosafety and stringent quality control measures in the wake of the pandemic has underscored the importance of mycoplasma testing in ensuring the integrity of research and manufacturing processes.
The biopharmaceutical industry in the United States has experienced significant growth, and this expansion has profoundly impacted the mycoplasma testing market. As the demand for biopharmaceutical products continues to rise, ensuring the safety and quality of these products becomes paramount, and mycoplasma testing plays a crucial role in this regard.
In recent years, the U.S. has witnessed a surge in the development and commercialization of advanced therapies, particularly in oncology, rare diseases, and regenerative medicine. These breakthroughs have expanded the biopharmaceutical industry and heightened the need for sophisticated mycoplasma testing methodologies capable of detecting minute contamination in complex biological products. The U.S. biopharmaceutical sector has witnessed a surge in research and development activities, leading to increased biologics production, cell and gene therapies, and vaccines. These advanced therapeutic modalities are highly sensitive to microbial contamination, making mycoplasma testing a critical step in the manufacturing process in the United States.
According to the Pharmaceutical Research and Manufacturers Association (PhRMA), in 2019, foreign direct investment in pharmaceuticals and medicines totaled $511.3 billion. The United States is the largest industry for biopharmaceuticals, accounting for around a third of the global industry, and is the world leader in biopharmaceutical R&D. With the U.S. already serving as the largest industry for biopharmaceuticals and a frontrunner in research and development within the sector, the mycoplasma testing market is poised to grow in tandem with the increasing demand for robust quality control measures.
Regulatory agencies in the United States, such as the Food and Drug Administration (FDA), emphasize the importance of robust testing procedures to ensure the safety of biopharmaceutical products. Moreover, the increasing trend toward outsourcing biopharmaceutical manufacturing processes to contract manufacturing organizations (CMOs) has fueled the demand for mycoplasma testing services. Therefore, the exponential growth of the U.S. biopharmaceutical industry is driven by advancements in therapeutic modalities and increased outsourcing to CMOs.
Adopting Polymerase Chain Reaction (PCR) technology in the United States has witnessed a significant upswing within the mycoplasma testing market. One key driver of the increasing adoption of PCR technology is the stringent regulatory requirements in the U.S. for ensuring the safety and quality of biological products. Real-time PCR, in particular, allows for continuous monitoring of the amplification process, enabling faster and more accurate detection of mycoplasma contamination. This technological evolution has made PCR a valuable tool for routine testing and quality control procedures in laboratories across the U.S.
Moreover, the rising investment in research and development activities within the biotechnology and pharmaceutical sectors in the U.S. has fueled the demand for reliable and rapid mycoplasma testing methods. Firstly, the pharmaceutical and biotechnology industries in the U.S. have experienced a substantial increase in the production of biologics, cell-based therapies, and other advanced therapeutic products. As these industries strive for excellence in product quality, the need for reliable mycoplasma testing methods has become paramount.
Additionally, the biopharmaceutical sector is witnessing a growing trend of outsourcing manufacturing processes to contract development and manufacturing organizations. These organizations play a crucial role in producing biologics for various clients. The advancements in PCR technology itself have contributed to its widespread adoption. Hence, the surge in PCR technology adoption for mycoplasma testing in the U.S. is driven by increased R&D investments in the biotechnology and pharmaceutical sectors and the growing trend of outsourcing to CDMOs, reflecting its pivotal role in ensuring product safety and quality.
The U.S. mycoplasma testing market is characterized by a diverse range of companies offering innovative solutions to ensure the detection and prevention of mycoplasma contamination. These companies are crucial in maintaining the integrity and quality of biopharmaceutical and cell culture products susceptible to mycoplasma contamination.
One prominent player in the U.S. mycoplasma testing market is ATCC (American Type Culture Collection). ATCC is a renowned biological resource center that provides a wide range of authenticated cell lines and microorganisms, including mycoplasma reference strains. Researchers and biopharmaceutical companies widely use the company's mycoplasma testing products to ensure the reliability of their cell cultures and biological products.
Bio-Rad Laboratories is another notable participant in the U.S. mycoplasma testing market. The company offers a variety of assays and testing kits for mycoplasma detection, focusing on delivering reliable and rapid results. Bio-Rad's products are widely utilized in both research and industrial settings, contributing to the overall integrity of cell cultures and biopharmaceutical manufacturing processes in the United States.
Another key contributor to the U.S. mycoplasma testing market is Lonza Group. Lonza offers a comprehensive portfolio of mycoplasma detection and elimination products catering to the specific needs of the biopharmaceutical industry. With a strong emphasis on quality control and regulatory compliance, Lonza's solutions are widely adopted by pharmaceutical companies and research institutions across the United States.
Additionally, Thermo Fisher Scientific plays a crucial role in the U.S. mycoplasma testing market by providing a comprehensive range of testing solutions. The company's mycoplasma detection products are designed to enhance the efficiency and accuracy of testing procedures, ensuring the safety and quality of biopharmaceutical products in the U.S. mycoplasma testing market. These companies contribute significantly to developing and advancing mycoplasma testing technologies, supporting the growing needs of the biopharmaceutical industry in the United States.
By Product & Service
By Technology
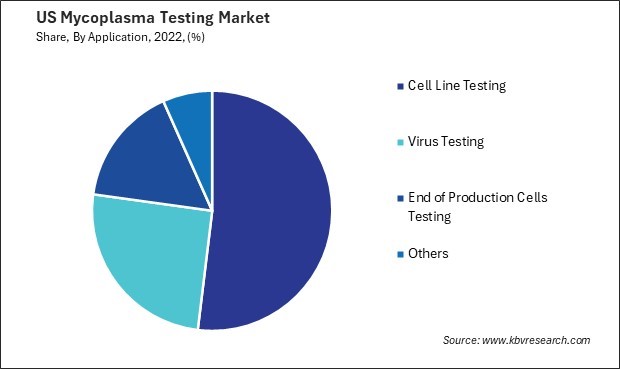
By Application
By End User
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

