US Mycoplasma Testing Market Size, Share & Trends Analysis Report By Product & Service (Kits & Reagents, Instruments and Services), By Technology, By Application, By End User, and Forecast, 2023 - 2030
Published Date : 20-May-2024 |
Pages: 76 |
Formats: PDF |
COVID-19 Impact on the US Mycoplasma Testing Market
The United States (US) Mycoplasma Testing Market size is expected to reach $541.8 Million by 2030, rising at a market growth of 10.1% CAGR during the forecast period.
The mycoplasma testing market in the United States has witnessed significant growth in recent years, driven by the increasing awareness and importance of mycoplasma contamination in cell culture processes, biopharmaceutical production, and research laboratories. One of the key factors contributing to the growth of the mycoplasma testing market in the U.S. is the rising adoption of cell-based research and biopharmaceutical manufacturing.
With the ongoing advancements in biotechnology and pharmaceutical research, the mycoplasma testing market in the U.S. is expected to continue its growth trajectory. The need for accurate and efficient testing solutions to ensure the integrity of cell cultures and biopharmaceutical products will remain a driving force, making the U.S. a significant hub for mycoplasma testing advancements and solutions. The U.S. mycoplasma testing market is characterized by several key players offering various testing kits, reagents, and services. These companies are focusing on innovations to enhance the sensitivity and specificity of their testing products. Furthermore, collaborations and partnerships between industry players and research institutions contribute to developing advanced testing methodologies.
As the biopharmaceutical industry in the U.S. continues to witness growth and innovation, the need for mycoplasma testing is expected to persist and expand. Emerging biotechnologies, such as cell and gene therapies, present new challenges and opportunities for mycoplasma testing, as these advanced therapies involve complex cell cultures. Consequently, the mycoplasma testing market in the U.S. is likely to witness sustained growth as these cutting-edge therapies become more prevalent in the healthcare landscape.
The outbreak of the COVID-19 pandemic has also left a significant impact on the mycoplasma testing market in the United States. The disruption caused by the pandemic has led to delays in research and development activities across various industries. With laboratories prioritizing COVID-19-related research, other essential areas, including mycoplasma testing, experienced a slowdown. However, the increased emphasis on biosafety and stringent quality control measures in the wake of the pandemic has underscored the importance of mycoplasma testing in ensuring the integrity of research and manufacturing processes.
Market Trends
Expansion of the biopharmaceutical industry
The biopharmaceutical industry in the United States has experienced significant growth, and this expansion has profoundly impacted the mycoplasma testing market. As the demand for biopharmaceutical products continues to rise, ensuring the safety and quality of these products becomes paramount, and mycoplasma testing plays a crucial role in this regard.
In recent years, the U.S. has witnessed a surge in the development and commercialization of advanced therapies, particularly in oncology, rare diseases, and regenerative medicine. These breakthroughs have expanded the biopharmaceutical industry and heightened the need for sophisticated mycoplasma testing methodologies capable of detecting minute contamination in complex biological products. The U.S. biopharmaceutical sector has witnessed a surge in research and development activities, leading to increased biologics production, cell and gene therapies, and vaccines. These advanced therapeutic modalities are highly sensitive to microbial contamination, making mycoplasma testing a critical step in the manufacturing process in the United States.
According to the Pharmaceutical Research and Manufacturers Association (PhRMA), in 2019, foreign direct investment in pharmaceuticals and medicines totaled $511.3 billion. The United States is the largest industry for biopharmaceuticals, accounting for around a third of the global industry, and is the world leader in biopharmaceutical R&D. With the U.S. already serving as the largest industry for biopharmaceuticals and a frontrunner in research and development within the sector, the mycoplasma testing market is poised to grow in tandem with the increasing demand for robust quality control measures.
Regulatory agencies in the United States, such as the Food and Drug Administration (FDA), emphasize the importance of robust testing procedures to ensure the safety of biopharmaceutical products. Moreover, the increasing trend toward outsourcing biopharmaceutical manufacturing processes to contract manufacturing organizations (CMOs) has fueled the demand for mycoplasma testing services. Therefore, the exponential growth of the U.S. biopharmaceutical industry is driven by advancements in therapeutic modalities and increased outsourcing to CMOs.
Increasing adoption of PCR technology
Adopting Polymerase Chain Reaction (PCR) technology in the United States has witnessed a significant upswing within the mycoplasma testing market. One key driver of the increasing adoption of PCR technology is the stringent regulatory requirements in the U.S. for ensuring the safety and quality of biological products. Real-time PCR, in particular, allows for continuous monitoring of the amplification process, enabling faster and more accurate detection of mycoplasma contamination. This technological evolution has made PCR a valuable tool for routine testing and quality control procedures in laboratories across the U.S.
Moreover, the rising investment in research and development activities within the biotechnology and pharmaceutical sectors in the U.S. has fueled the demand for reliable and rapid mycoplasma testing methods. Firstly, the pharmaceutical and biotechnology industries in the U.S. have experienced a substantial increase in the production of biologics, cell-based therapies, and other advanced therapeutic products. As these industries strive for excellence in product quality, the need for reliable mycoplasma testing methods has become paramount.
Additionally, the biopharmaceutical sector is witnessing a growing trend of outsourcing manufacturing processes to contract development and manufacturing organizations. These organizations play a crucial role in producing biologics for various clients. The advancements in PCR technology itself have contributed to its widespread adoption. Hence, the surge in PCR technology adoption for mycoplasma testing in the U.S. is driven by increased R&D investments in the biotechnology and pharmaceutical sectors and the growing trend of outsourcing to CDMOs, reflecting its pivotal role in ensuring product safety and quality.
Competition Analysis
The U.S. mycoplasma testing market is characterized by a diverse range of companies offering innovative solutions to ensure the detection and prevention of mycoplasma contamination. These companies are crucial in maintaining the integrity and quality of biopharmaceutical and cell culture products susceptible to mycoplasma contamination.
One prominent player in the U.S. mycoplasma testing market is ATCC (American Type Culture Collection). ATCC is a renowned biological resource center that provides a wide range of authenticated cell lines and microorganisms, including mycoplasma reference strains. Researchers and biopharmaceutical companies widely use the company's mycoplasma testing products to ensure the reliability of their cell cultures and biological products.
Bio-Rad Laboratories is another notable participant in the U.S. mycoplasma testing market. The company offers a variety of assays and testing kits for mycoplasma detection, focusing on delivering reliable and rapid results. Bio-Rad's products are widely utilized in both research and industrial settings, contributing to the overall integrity of cell cultures and biopharmaceutical manufacturing processes in the United States.
Another key contributor to the U.S. mycoplasma testing market is Lonza Group. Lonza offers a comprehensive portfolio of mycoplasma detection and elimination products catering to the specific needs of the biopharmaceutical industry. With a strong emphasis on quality control and regulatory compliance, Lonza's solutions are widely adopted by pharmaceutical companies and research institutions across the United States.
Additionally, Thermo Fisher Scientific plays a crucial role in the U.S. mycoplasma testing market by providing a comprehensive range of testing solutions. The company's mycoplasma detection products are designed to enhance the efficiency and accuracy of testing procedures, ensuring the safety and quality of biopharmaceutical products in the U.S. mycoplasma testing market. These companies contribute significantly to developing and advancing mycoplasma testing technologies, supporting the growing needs of the biopharmaceutical industry in the United States.
List of Key Companies Profiled
- Charles River Laboratories International, Inc.
- Thermo Fisher Scientific, Inc.
- Eurofins Scientific SE
- Lonza Group Ltd.
- Bio-Rad laboratories, Inc.
- InvivoGen SAS
- Asahi Kasei Corporation
- F.Hoffmann-La Roche Ltd.
- Norgen Biotek Corp.
- PromoCell GmbH
US Mycoplasma Testing Market Report Segmentation
By Product & Service
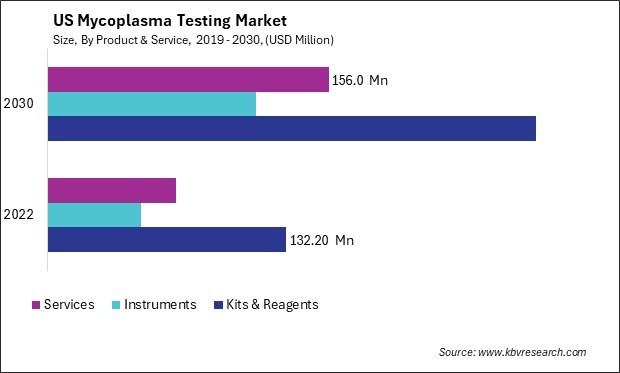
- Kits & Reagents
- Instruments
- Services
By Technology
- PCR
- ELISA
- Microbial Culture Techniques
- Enzymatic Methods
By Application
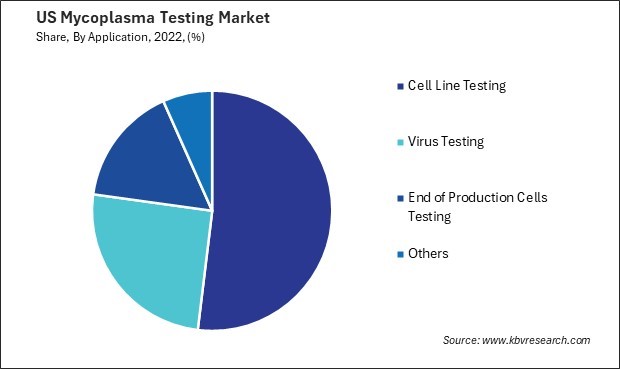
- Cell Line Testing
- Virus Testing
- End of Production Cells Testing
- Others
By End User
- Pharmaceutical & Biotechnology Companies
- Contract Research Organizations
- Academic Research Institutes
- Cell Banks
- Others
1.1 Market Definition
1.2 Objectives
1.3 Market Scope
1.4 Segmentation
1.4.1 US Mycoplasma Testing Market, by Product & Service
1.4.2 US Mycoplasma Testing Market, by Technology
1.4.3 US Mycoplasma Testing Market, by Application
1.4.4 US Mycoplasma Testing Market, by End User
1.5 Methodology for the research
Chapter 2. Market Overview
2.1 Introduction
2.1.1 Overview
2.1.1.1 Market Composition and Scenario
2.2 Key Factors Impacting the Market
2.2.1 Market Drivers
2.2.2 Market Restraints
2.2.3 Market Trends
2.3 Porter Five Forces Analysis
Chapter 3. US Mycoplasma Testing Market
3.1 US Mycoplasma Testing Market by Product & Service
3.2 US Mycoplasma Testing Market by Technology
3.3 US Mycoplasma Testing Market by Application
3.4 US Mycoplasma Testing Market by End User
Chapter 4. Company Profiles – Global Leaders
4.1 Charles River Laboratories International, Inc.
4.1.1 Company Overview
4.1.2 Financial Analysis
4.1.3 Segmental and Regional Analysis
4.2 Thermo Fisher Scientific, Inc.
4.2.1 Company Overview
4.2.2 Financial Analysis
4.2.3 Segmental and Regional Analysis
4.2.4 Research & Development Expenses
4.2.5 SWOT Analysis
4.3 Eurofins Scientific SE
4.3.1 Company Overview
4.3.2 Financial Analysis
4.3.3 Regional Analysis
4.3.4 Recent strategies and developments:
4.3.4.1 Product Launches and Product Expansions:
4.3.4.2 Acquisition and Mergers:
4.3.5 SWOT Analysis
4.4 Lonza Group Ltd.
4.4.1 Company Overview
4.4.2 Financial Analysis
4.4.3 Segmental and Regional Analysis
4.4.4 Research & Development Expenses
4.4.5 SWOT Analysis
4.5 Bio-Rad laboratories, Inc.
4.5.1 Company Overview
4.5.2 Financial Analysis
4.5.3 Segmental and Regional Analysis
4.5.4 Research & Development Expenses
4.5.5 SWOT Analysis
4.6 InvivoGen SAS
4.6.1 Company Overview
4.6.2 SWOT Analysis
4.7 Asahi Kasei Corporation
4.7.1 Company Overview
4.7.2 Financial Analysis
4.7.3 Segmental and Regional Analysis
4.7.4 Research & Development Expenses
4.7.5 SWOT Analysis
4.8 F. Hoffmann-La Roche Ltd.
4.8.1 Company Overview
4.8.2 Financial Analysis
4.8.3 Segmental and Regional Analysis
4.8.4 Research & Development Expense
4.8.5 SWOT Analysis
4.9 Norgen Biotek Corp.
4.9.1 Company Overview
4.10. PromoCell GmbH
4.10.1 Company Overview
4.10.2 SWOT Analysis
TABLE 2 US Mycoplasma Testing Market, 2023 - 2030, USD Million
TABLE 3 US Mycoplasma Testing Market by Product & Service, 2019 - 2022, USD Million
TABLE 4 US Mycoplasma Testing Market by Product & Service, 2023 - 2030, USD Million
TABLE 5 US Mycoplasma Testing Market by Technology, 2019 - 2022, USD Million
TABLE 6 US Mycoplasma Testing Market by Technology, 2023 - 2030, USD Million
TABLE 7 US Mycoplasma Testing Market by Application, 2019 - 2022, USD Million
TABLE 8 US Mycoplasma Testing Market by Application, 2023 - 2030, USD Million
TABLE 9 US Mycoplasma Testing Market by End User, 2019 - 2022, USD Million
TABLE 10 US Mycoplasma Testing Market by End User, 2023 - 2030, USD Million
TABLE 11 Key Information – Charles River Laboratories International, Inc.
TABLE 12 Key Information – Thermo Fisher Scientific, Inc.
TABLE 13 Key Information – Eurofins Scientific SE
TABLE 14 Key Information –Lonza Group Ltd.
TABLE 15 Key Information – Bio-Rad Laboratories, Inc.
TABLE 16 Key Information – InvivoGen SAS
TABLE 17 Key Information – Asahi Kasei Corporation
TABLE 18 Key Information – F. Hoffmann-La Roche Ltd.
TABLE 19 Key Information – Norgen Biotek Corp.
TABLE 20 Key Information – PromoCell GmbH
List of Figures
FIG 1 Methodology for the research
FIG 2 US Mycoplasma Testing Market, 2019 - 2030, USD Million
FIG 3 Key Factors Impacting Mycoplasma Testing Market
FIG 4 Porter’s Five Forces Analysis – Mycoplasma Testing Market
FIG 5 US Mycoplasma Testing Market share by Product & Service, 2022
FIG 6 US Mycoplasma Testing Market share by Product & Service, 2030
FIG 7 US Mycoplasma Testing Market by Product & Service, 2019 - 2030, USD Million
FIG 8 US Mycoplasma Testing Market share by Technology, 2022
FIG 9 US Mycoplasma Testing Market share by Technology, 2030
FIG 10 US Mycoplasma Testing Market by Technology, 2019 - 2030, USD Million
FIG 11 US Mycoplasma Testing Market share by Application, 2022
FIG 12 US Mycoplasma Testing Market share by Application, 2030
FIG 13 US Mycoplasma Testing Market by Application, 2019 - 2030, USD Million
FIG 14 US Mycoplasma Testing Market share by End User, 2022
FIG 15 US Mycoplasma Testing Market share by End User, 2030
FIG 16 US Mycoplasma Testing Market by End User, 2019 - 2030, USD Million
FIG 17 SWOT Analysis: Thermo Fisher Scientific, Inc.
FIG 18 SWOT Analysis: Eurofins Scientific SE
FIG 19 SWOT Analysis: Lonza Group Ltd.
FIG 20 SWOT Analysis: Bio-Rad Laboratories, Inc.
FIG 21 SWOT Analysis: InvivoGen SAS
FIG 22 SWOT Analysis: Asahi Kasei Corporation
FIG 23 SWOT Analysis: F. Hoffmann-La Roche Ltd.
FIG 24 SWOT Analysis: PromoCell GmbH