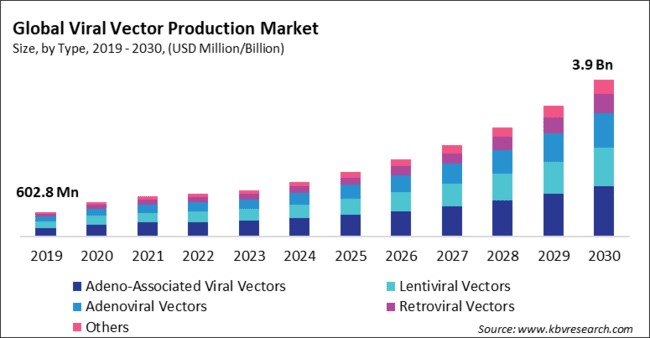
“Global Viral Vector Production Market to reach a market value of USD 3.9 Billion by 2030 growing at a CAGR of 19.1%”
The Global Viral Vector Production Market size is expected to reach $3.9 billion by 2030, rising at a market growth of 19.1% CAGR during the forecast period.
This has created a higher demand for viral vectors in producing these therapies. Regulatory agencies recognizing the potential of gene therapies and offering support, expedited review processes, and incentives have contributed to the growth of the gene therapy. Consequently, the Gene Therapy segment would generate approximately 65.6 share of the market by 2030. This positive regulatory environment has encouraged the development and production of viral vectors. Thus, the rise in demand and awareness for gene therapy has positively impacted the market by creating a growing and dynamic landscape.
Engineering viral vectors for reduced immunogenicity, improved targeting, and controlled gene expression contributes to developing vectors tailored for specific therapeutic applications. Developing next-generation viral vector platforms, such as synthetic and hybrid vectors, expands the toolbox for gene delivery. These platforms aim to overcome limitations associated with traditional vectors, offering improved safety profiles and increased efficiency in gene transfer. Therefore, these advancements collectively drive the expansion of the market. Moreover, A growing pipeline in gene therapy addresses a diverse range of therapeutic targets, including genetic disorders, rare diseases, neurodegenerative conditions, and certain types of cancer. This diversity broadens the application of viral vectors, leading to increased production needs. The expansion of clinical trials in gene therapy and viral vaccines, driven by a robust pipeline, necessitates large-scale production of viral vectors for investigational treatments. Due to the above aspects, the market is expected to grow significantly.
Furthermore, Viral vector platforms, particularly adenoviruses, had been instrumental in developing several COVID-19 vaccines. This surge in demand had led to an increased focus on optimizing viral vector production processes to meet the global need for large-scale vaccine manufacturing. The pandemic had prompted an unprecedented acceleration of research and development efforts in the biopharmaceutical sector. This emphasis on equitable distribution had implications for the manufacturing capacity and scalability of viral vector production, driving innovations in production processes to meet the demands of a global vaccination effort. Thus, COVID-19 had a positive impact on the market.
However, Viral vector production involves complex and intricate processes, including cell culture, transfection, purification, and quality control. The complexity of these processes contributes to increased production costs, particularly in terms of skilled labor, specialized equipment, and consumables. Also, the production of viral vectors often requires specialized facilities with controlled environments to ensure the integrity and safety of the final product. Due to the above factors, market growth will be hampered in the coming years.
By type, the market is classified into adenoviral vectors, lentiviral vectors, retroviral vectors, adeno-associated viral vectors, and others. In 2022, the adeno-associated viral vectors segment registered the highest revenue share in the market. Adeno-associated viral vectors (AAVs) typically induce a minimal immune response, allowing for repeated administration without significant immune reactions. This low immunogenicity is advantageous for long-term and repeated treatments. AAVs can establish stable and long-lasting gene expression in dividing and non-dividing cells. This property is crucial for sustained therapeutic effects and reduces the need for frequent vector administration. They can infect both dividing and non-dividing partitions, making them versatile for targeting various cell types, including neurons, muscle cells, and liver cells.
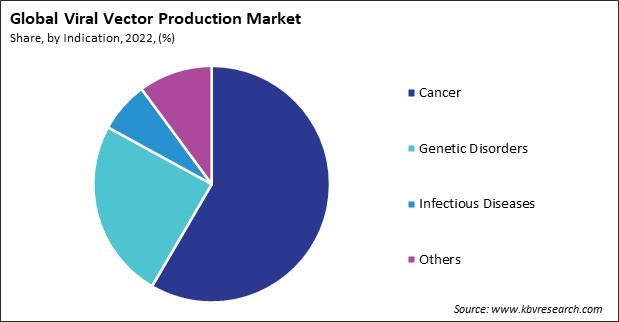
On the basis of indication, the market is segmented into cancer, genetic disorders, infectious diseases, and others. The genetic disorders segment acquired a substantial revenue share in the market in 2022. Viral vectors deliver therapeutic genes into target cells in patients with genetic disorders. The viral vector works as a delivery vehicle, carrying the corrected or functional gene to the cells affected by the genetic abnormality. This approach aims to replace or supplement the defective gene, addressing the root cause of the genetic disorder. Genetic disorders caused by enzyme deficiencies, such as lysosomal storage disorders, can be targeted using viral vectors. Introducing functional genes encoding missing enzymes into affected cells can help restore enzymatic activity and alleviate the accumulation of substrates that lead to disease symptoms.
Based on application, the market is fragmented into gene therapy and vaccinology. In 2022, the gene therapy segment held the highest revenue share in the market. Gene therapy can be applied using in vivo or ex vivo approaches. In in vivo gene therapy, viral vectors are directly administered to the patient to target specific tissues or organs. Ex vivo gene therapy involves modifying cells outside the patient's body before reinfusing them. The preference of approach depends on the target tissue and the therapeutic strategy. The increasing number of clinical trials and regulatory approvals is evidence of the success of viral vector-based gene therapies. Positive outcomes in clinical studies contribute to validating and accepting gene therapy as a viable treatment option for genetic disorders.
Free Valuable Insights: Global Viral Vector Production Market size to reach USD 3.9 Billion by 2030
Region-wise, the market is analysed across North America, Europe, Asia Pacific, and LAMEA. The Asia Pacific region covered a considerable revenue share in the market in 2022. In recent years, the Asia-Pacific region has witnessed substantial growth in the biotechnology and pharmaceutical sectors. This growth is propelled by increasing investment in research and development, expanding healthcare infrastructure, and increasing government support for the biotech industry. As a result, there is a growth in demand for viral vectors in the region to support the development and production of advanced therapies. In addition, the Asia-Pacific region has a high hindrance of cancer, genetic disorders, and infectious diseases. As a result, there is an increased focus on developing gene therapies and viral vector-based vaccines to address these healthcare challenges. The demand for viral vectors as essential tools in developing therapies drives the growth of the market.
| Report Attribute | Details |
|---|---|
| Market size value in 2022 | USD 1 Billion |
| Market size forecast in 2030 | USD 3.9 Billion |
| Base Year | 2022 |
| Historical Period | 2019 to 2021 |
| Forecast Period | 2023 to 2030 |
| Revenue Growth Rate | CAGR of 19.1% from 2023 to 2030 |
| Number of Pages | 232 |
| Number of Tables | 370 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Porter’s 5 Forces Analysis, Company Profiling, Companies Strategic Developments, SWOT Analysis, Winning Imperatives |
| Segments covered | Type, Indication, Application, Region |
| Country scope |
|
| Companies Included | Andelyn Biosciences, Inc., Charles River Laboratories International, Inc. Danaher Corporation, FinVector Oy (Ferring Ventures S/A), Thermo Fisher Scientific, Inc., Novartis AG, Takara Bio Inc. (Takara Holdings Inc.)Avid Bioservices, Inc., Oxford Biomedica plc, Lonza Group Ltd. |
By Type
By Indication
By Application
By Geography
The Market size is projected to reach USD $3.9 billion by 2030.
Robust pipeline in gene therapy and viral vaccines are driving the Market in coming years, however, Regulatory requirements for viral vector production restraints the growth of the Market.
Andelyn Biosciences, Inc., Charles River Laboratories International, Inc. Danaher Corporation, FinVector Oy (Ferring Ventures S/A), Thermo Fisher Scientific, Inc., Novartis AG, Takara Bio Inc. (Takara Holdings Inc.)Avid Bioservices, Inc., Oxford Biomedica plc, Lonza Group Ltd.
The expected CAGR of this Market is 19.1% from 2023 to 2030.
The Cancer segment is leading the Market, by Indication in 2022 thereby, achieving a market value of $2.1 Billion by 2030.
The North America region dominated the Market, by region in 2022, and would continue to be a dominant market till 2030; thereby, achieving a market value of $1.6 Billion by 2030, growing at a CAGR of 18 % during the forecast period.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

 Drivers
Drivers
 Restraints
Restraints
